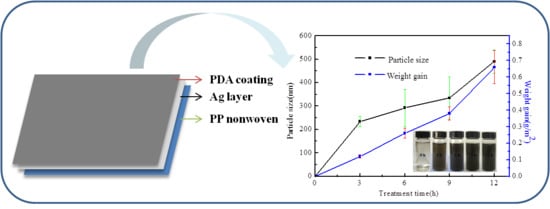
The Effect of Polydopamine on an Ag-Coated Polypropylene Nonwoven Fabric
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Magnetron Sputtering
2.3. Deposition of PDA on PP Nonwoven Fabrics
2.4. Characterization
2.5. Stability
2.6. Durability
3. Results and Discussion
3.1. Morphology and Chemical Composition Analysis
3.2. EDS Analysis
3.3. Electrical Conductivity Analysis
3.4. Electromagnetic Interference Shielding Effectiveness Analysis
3.5. Antibacterial Activity Analysis
3.6. Stability Analysis
3.7. Laundering Durability Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kang, S.M.; Rho, J.; Choi, I.S.; Messersmith, P.B.; Lee, H. Norepinephrine: Material-independent, multifunctional surface modification reagent. J. Am. Chem. Soc. 2009, 131, 13224–13225. [Google Scholar] [CrossRef]
- Geng, A.; Meng, L.; Han, J.; Zhong, Q.; Li, M.; Han, S.; Mei, C.; Xu, L.; Tan, L.; Gan, L. Highly efficient visible-light photocatalyst based on cellulose derived carbon nanofiber/biobr composites. Cellulose 2018, 25, 4133–4144. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Wang, R.; Lu, H.; Li, L.; Kong, Y.; Qi, K.; Xin, J.H. Artificial lotus leaf structures from assembling carbon nanotubes and their applications in hydrophobic textiles. J. Mater. Chem. 2007, 17, 1071–1078. [Google Scholar] [CrossRef]
- Guo, R.H.; Jiang, S.X.; Yuen, C.W.; Lan, J.W. Optimization of electroless nickel plating on polyester fabric. Fibers Polym. 2013, 14, 459–464. [Google Scholar] [CrossRef]
- Yip, J.; Jiang, S.; Wong, C. Characterization of metallic textiles deposited by magnetron sputtering and traditional metallic treatments. Surf. Coat. Technol. 2009, 204, 380–385. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Y.; Liu, X.Q. Polymer interface molecular engineering for E-Textiles. Polymers 2018, 10, 573. [Google Scholar] [CrossRef]
- Brozena, A.H.; Oldham, C.J.; Parsons, G.N. Atomic layer deposition on polymer fibers and fabrics for multifunctional and electronic textiles. J. Vac. Sci. Technol. 2016, 34, 010801. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Zhao, Y. One-step fabrication of robust fabrics with both-faced superhydrophobicity for the separation and capture of oil from water. Phys. Chem. Chem. Phys. 2015, 17, 6451–6457. [Google Scholar] [CrossRef]
- Wang, T.; Liu, G.; Kong, J. Preparation of wood-like structured copper with superhydrophobic properties. Sci Rep. 2015, 5, 18328. [Google Scholar] [CrossRef]
- Nagappan, S.; Ha, C.S. Emerging trends in superhydrophobic surface based magnetic materials: Fabrications and their potential applications. J. Mater. Chem. 2015, 3, 3224–3251. [Google Scholar] [CrossRef]
- Jiang, S.X.; Qin, W.F.; Xu, M.T. Surface characterization of sputter silver-coated polyester fiber. Fibers Polym. 2011, 12, 616–619. [Google Scholar] [CrossRef]
- Bobzin, K.; Bagcivan, N.; Immich, P. Advantages of nanocomposite coatings deposited by high power pulse magnetron sputtering technology. J. Mater. Process. Technol. 2009, 2009, 165–170. [Google Scholar] [CrossRef]
- Jiang, S.; Miao, D.; Zhao, D. Adhesive properties of SS to PU and PVC leathers. Int. J. Cloth. Sci. Technol. 2014, 26, 108–117. [Google Scholar] [CrossRef]
- Jiang, S.; Miao, D.; Li, A.; Guo, R.; Shang, S. Adhesion and durability of Cu film on polyester fabric prepared by finishing treatment with polyester-polyurethane and aqueous acrylate. Fibers Polym. 2016, 17, 1397–1402. [Google Scholar] [CrossRef]
- Bajaj, P. Finishing of textile materials. J. Appl. Polym. Sci. 2010, 83, 631–659. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-Molecule Mechanics of Mussel Adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef]
- Wilker, J.J. Positive charges and underwater adhesion. Science 2015, 349, 582–583. [Google Scholar] [CrossRef]
- Kord, F.P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Ryu, J.; Ku, S.H.; Lee, H. Mussel-Inspired Polydopamine Coating as a Universal Route to Hydroxyapatite Crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Xi, Z.Y.; Xu, Y.Y.; Zhu, L.P. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Yang, S.H.; Kang, S.M.; Lee, K.B. Mussel-inspired encapsulation and functionalization of individual yeast cells. J. Am. Chem. Soc. 2011, 133, 2795–2797. [Google Scholar] [CrossRef]
- Ku, S.H.; Ryu, J.; Hong, S.K. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef]
- Zhu, C.; Guan, X.Y.; Wang, X.; Li, Y.; Chalmers, E. Mussel-Inspired Flexible, Durable, and Conductive Fibers Manufacturing for Finger-Monitoring Sensors. Adv. Mater. Interfaces 2019, 6, 1801547. [Google Scholar] [CrossRef]
- Ryou, M.H.; Yong, M.L.; Park, J.K. Mussel-Inspired Polydopamine-Treated Polyethylene Separators for High-Power Li-Ion Batteries. Adv. Mater. 2011, 23, 3066–3070. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.C.; Wan, L.S. Polydopamine-Coated Porous Substrates as a Platform for Mineralized β-FeOOH Nanorods with Photocatalysis under Sunlight. ACS Appl. Mater. Interfaces 2015, 7, 11567–11574. [Google Scholar] [CrossRef]
- Chao, Z.; Yan, L.; Qiu, W.Z.; He, A.; Xu, Z.K. Polydopamine Coatings with Nanopores for Versatile Molecular Separation. ACS Appl. Mater. Interfaces 2017, 9, 14437–14444. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Jiang, L.; Guo, Y.; Sun, Z.H. Rapid fabrication of silver nanoparticle /polydopamine functionalized polyester fibers. Text. Res. J. 2019. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, Y.; Wen, S. Preparation and characterization of polystyrene/Ag core-shell microspheres—A bio-inspired poly(dopamine) approach. J. Colloid Interface Sci. 2012, 368, 241–249. [Google Scholar] [CrossRef]
- Ma, Z.; Jia, X.; Hu, J. Mussel-inspired chemistry for one-step synthesis of N-doped carbon–gold composites with morphology tailoring and their catalytic properties. RSC Adv. 2013, 4, 1853–1856. [Google Scholar] [CrossRef]
- Kim, H.C.; Theodore, N.D.; Alford, T.L. Comparison of texture evolution in Ag and Ag(Al) alloy thin films on amorphous SiO2. J. Appl. Phys. 2004, 95, 5180–5188. [Google Scholar] [CrossRef]
- Sahu, D.R.; Huang, J.L. High quality transparent conductive ZnO/Ag/ZnO multilayer films deposited at room temperature. Thin Solid Films 2006, 515, 876–879. [Google Scholar] [CrossRef]
- Kim, H.C.; Alford, T.L. Improvement of the thermal stability of silver metallization. J. Appl. Phys. 2003, 94, 5393–5395. [Google Scholar] [CrossRef]
- Yu, B.; Wang, D.A.; Ye, Q. Robust polydopamine nano/microcapsules and their loading and release behavior. Chem. Commun. 2009, 44, 6789–6791. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Asiri, A.M.; Abdelwahed, N.A.M. In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 2011, 18, 75–82. [Google Scholar] [CrossRef]
- Franey, J.P.; Kammlott, G.W.; Graedel, T.E. The corrosion of silver by atmospheric sulfurous gases. Corros. Sci. 1985, 25, 133–143. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-Inspired Materials: Self-Healing through Coordination Chemistry. Chemistry 2016, 22, 844–857. [Google Scholar] [CrossRef]














| Film | Base Pressure | DC Power | Ar Flow Rate | Working Pressure | Thickness |
|---|---|---|---|---|---|
| Ag | 5.0 × 10−4 Pa | 100 W | 20 sccm | 3.0 × 10−1 Pa | 100 nm |
| PDA Treatment Time (h) | 0 | 3 | 6 | 9 | 12 |
|---|---|---|---|---|---|
| R (Ω/sq) | 5.5 | 6.75 | 10.3 | 12.5 | 12.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, J.; Ning, X.; Chen, S.; Liu, Z.; Jiang, S.; Miao, D. The Effect of Polydopamine on an Ag-Coated Polypropylene Nonwoven Fabric. Polymers 2019, 11, 627. https://doi.org/10.3390/polym11040627
Liu C, Liu J, Ning X, Chen S, Liu Z, Jiang S, Miao D. The Effect of Polydopamine on an Ag-Coated Polypropylene Nonwoven Fabric. Polymers. 2019; 11(4):627. https://doi.org/10.3390/polym11040627
Chicago/Turabian StyleLiu, Chuanmei, Jie Liu, Xin Ning, Shaojuan Chen, Zhengqin Liu, Shouxiang Jiang, and Dagang Miao. 2019. "The Effect of Polydopamine on an Ag-Coated Polypropylene Nonwoven Fabric" Polymers 11, no. 4: 627. https://doi.org/10.3390/polym11040627
APA StyleLiu, C., Liu, J., Ning, X., Chen, S., Liu, Z., Jiang, S., & Miao, D. (2019). The Effect of Polydopamine on an Ag-Coated Polypropylene Nonwoven Fabric. Polymers, 11(4), 627. https://doi.org/10.3390/polym11040627