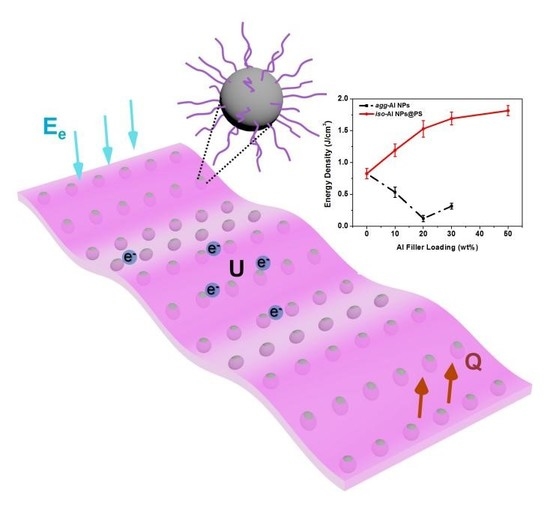
Polymer Grafted Aluminum Nanoparticles for Percolative Composite Films with Enhanced Compatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
2.2. Preparation of Al Nanoparticles
2.3. PS Grafting to Al NPs
2.4. Preparation of the PS Film Doped with Al NPs
2.5. Humidity Assay
2.6. Measurement of Dielectric Constant
2.7. Measurement of Breakdown Strength
2.8. Characterization and Instruments
3. Results and Discussion
3.1. Preparation and Characterization of Al NPs Doped PS Composite Films
3.2. Dielectric Properties of Al NPs Doped PS Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brousseau, P.; Anderson, C.J. Nanometric Aluminum in Explosives. Propellants Explos. Pyrotech. 2002, 27, 300–306. [Google Scholar] [CrossRef]
- Pivkina, A.; Ulyanova, P.; Frolov, Y.; Zavyalov, S.; Schoonman, J. Nanomaterials for Heterogeneous Combustion. Propellants Explos. Pyrotech. 2004, 29, 39–48. [Google Scholar] [CrossRef]
- Malchi, J.Y.; Foley, T.J.; Yetter, R.A. Electrostatically Self-Assembled Nanocomposite Reactive Microspheres. ACS Appl. Mater. Interfaces 2009, 1, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Levitas, V.I.; Asay, B.W.; Son, S.F.; Pantoya, M. Mechanochemical mechanism for fast reaction of metastable intermolecular composites based on dispersion of liquid metal. J. Appl. Phys. 2007, 101, 083524. [Google Scholar] [CrossRef]
- Sun, J.; Pantoya, M.L.; Simon, S.L. Dependence of size and size distribution on reactivity of aluminum nanoparticles in reactions with oxygen and MoO3. Thermochim. Acta 2007, 101, 083524. [Google Scholar] [CrossRef]
- Pantoya, M.L.; Granier, J. Combustion Behavior of Highly Energetic Thermites: Nano versus Micron Composites. Propellants Explos. Pyrotech. 2005, 30, 53–62. [Google Scholar] [CrossRef]
- DeLuca, L.T.; Galfetti, L.; Colombo, G.; Maggi, F.; Bandera, A. Microstructure Effects in Aluminized Solid Rocket Propellants. J. Propul. Power 2010, 26, 724–733. [Google Scholar] [CrossRef]
- Galfetti, L.; DeLuca, L.T.; DeLuca, F.; Meda, L.; Marra, G.; Marchetti, M.; Regi, M.; Belluci, S. Nanoparticles for Solid Rocket Propulsion. J. Phys. Condens. Matter 2006, 18, S1991–S2005. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Oldenburg, S.J.; Inman, A.O. Interactions of aluminum nanoparticles with human epidermal keratinocytes. J. Appl. Toxicl. 2010, 30, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, L.; Lian, Z.; Cao, M.; Li, H.; Sun, W.; Tong, N. Deep-Ultraviolet–Blue-Light Surface Plasmon Resonance of Al and Alcore/Al2O3shell in Spherical and Cylindrical Nanostructures. J. Phys. Chem. C 2012, 116, 15584–15590. [Google Scholar] [CrossRef]
- Knight, M.W.; King, N.S.; Liu, L.; Everitt, H.O.; Nordlander, P. Aluminum for Plasmonics. ACS Nano 2014, 8, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.W.; Liu, L.; Wang, Y.; Brown, L.; Mukherjee, S.; King, N.S.; Everitt, H.O.; Nordlander, P.; Halas, N.J. Aluminum Plasmonic Nanoantennas. Nano Lett. 2012, 12, 6000–6004. [Google Scholar] [CrossRef] [PubMed]
- Schade, M.; Fuhrmann, B.; Bohley, C.; Schlenker, S.; Sardana, N.; Schilling, J.; Leipner, H.S. Regular arrays of Al nanoparticles for plasmonic applications. J. Appl. Phys. 2014, 115, 205–213. [Google Scholar] [CrossRef]
- Lu, S.Y.; Yu, H.; Gottheim, S.; Gao, H.; DeSantis, C.J.; Clark, B.D.; Yang, J.; Jacobson, C.R.; Lu, Z.Y.; Nordlander, P.; et al. Polymer-Directed Growth of Plasmonic Aluminum Nanocrystals. J. Am. Chem. Soc. 2018, 140, 15412–15418. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Gromov, A.A.; Gromov, A.P.; Rim, G.H. Passivation Process for Superfine Aluminum Powders Obtained by Electrical Explosion of Wires. Appl. Sur. Sci. 2003, 211, 57–67. [Google Scholar] [CrossRef]
- Brege, J.J.; Hamilton, C.E.; Crouse, C.A.; Barron, A.R. Ultrasmall copper nanoparticles from a hydrophobically immobilized surfactant template. Nano Lett. 2009, 9, 2239–2242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Huo, P.F.; Wang, J.F.; Liu, X.; Rong, C.R.; Wang, G.B. Dielectric percolative composites with high dielectric constant and low dielectric loss based on sulfonated poly(aryl ether ketone) and a-MWCNTs coated with polyaniline. J. Mater. Chem. C 2013, 1, 4035–4041. [Google Scholar] [CrossRef]
- Lu, J.X.; Moon, K.S.; Wong, C.P. Silver/Polymer Nanocomposite as a High-Kpolymer Matrix for Dielectric Composites with Improved Dielectric Performance. J. Mater. Chem. 2008, 18, 4821–4826. [Google Scholar] [CrossRef]
- Dang, Z.M.; Peng, B.; Xie, D.; Yao, S.H.; Jiang, M.J.; Bai, J.B. High Dielectric Permittivity Silver/Polyimide Composite Films with Excellent Thermal Stability. Appl. Phys. Lett. 2008, 92, 112910. [Google Scholar] [CrossRef]
- Pecharroman, C.; Moya, J. Experimental Evidence of a Giant Capacitance in Insulator-Conductor Composites at the Percolation Threshold. Adv. Mater. 2000, 12, 294–297. [Google Scholar] [CrossRef]
- Wang, L.; Dang, Z.M. Carbon Nanotube Composites with High Dielectric Constant at Low Percolation Threshold. Appl. Phys. Lett. 2005, 87, 042903. [Google Scholar] [CrossRef]
- Dang, Z.M.; Lin, Y.H.; Nan, C.W. Novel Ferroelectric Polymer Composites with High Dielectric Constants. Adv. Mater. 2003, 85, 1625–1629. [Google Scholar] [CrossRef]
- Sreekumar, T.V.; Liu, T.; Min, B.G.; Guo, H.; Kumar, S.; Hauge, R.H.; Hauge, R.E. Polyacrylonitrile Single-Walled Carbon Nanotube Composite Fibers. Adv. Mater. 2004, 16, 58–61. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, T.S.; Choi, H.D.; Kwon, J.H.; Chung, Y.C. Electrical Conductivity of Chemically Modified Multiwalled Carbon Nanotube/Epoxy Composites. Carbon 2005, 43, 23–30. [Google Scholar] [CrossRef]
- Li, S.; Qin, Y.; Shi, J.; Guo, Z.-X.; Li, Y.; Zhu, D. Electrical Properties of Soluble Carbon Nanotube/Polymer Composite Films. Chem. Mater. 2005, 17, 130–135. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Zhu, M.; Zhu, X.; Rong, C.; Wang, G. Study on Novel Carbon-Nanotube/Sulfonated Poly(Aryl Ether Ketone) Composites with High Dielectric Constant at Low Percolation Threshold. Soft Mater. 2010, 9, 94–103. [Google Scholar] [CrossRef]
- Chung, D.D.L. Electrical Applications of Carbon Materials. J. Mater. Sci. 2004, 39, 2645–2661. [Google Scholar] [CrossRef]
- Xu, H.-P.; Dang, Z.-M.; Jiang, M.-J.; Yao, S.-H.; Bai, J.-B. Enhanced Dielectric Properties and Positive Temperature Coefficient Effect in the Binary Polymer Composites with Surface Modified Carbon Black. J. Mater. Chem. 2008, 18, 229–234. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, X.; Wang, W.; Wang, Z.; Song, M.; Wang, Y.; Wang, C. Fabrication of novel Ag nanowires/poly(vinylidene fluoride) nanocomposite film with high dielectric constant. Phys. Status Solidi A 2010, 207, 1870–1873. [Google Scholar] [CrossRef]
- Thomas, P.; Varughese, K.T.; Dwarakanath, K.; Varma, K.B.R. Dielectric properties of Poly(vinylidene fluoride)/CaCuTiO composites. Compos. Sci. Technol. 2010, 70, 539–545. [Google Scholar] [CrossRef]
- Xu, H.; Bai, Y.; Bharti, V.; Cheng, Z.-Y. High dielectric constant composites based on metallophthalocyanine oligomer and poly(vinylidene fluoride-trifluoroethylene) copolymer. J. Appl. Polym. Sci. 2001, 82, 70–75. [Google Scholar] [CrossRef]
- Wang, J.W.; Bao, H.M.; Yang, C.Z.; Shen, Q.D. High dielectric constant composite of P(VDF-TrFE) with grafted copper phthalocyanine oligomer. Macromolecules 2004, 37, 2294–2298. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Q.M. Fully Functionalized High-Dielectric-Constant Nanophase Polymers with High Electromechanical Response. Adv. Mater. 2005, 17, 1153–1158. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Q.M. Enhanced Dielectric and Electromechanical Responses in High Dielectric Constant All-Polymer Percolative Composites. Adv. Funct. Mater. 2004, 14, 501–506. [Google Scholar] [CrossRef]
- Exarhos, M.F.; Trudeau, M.L.; Alamdari, H.D.; Boily, S. Introductory Remarks on Nanodielectrics. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 808–818. [Google Scholar]
- Qi, L.; Lee, B.I.; Chen, S.; Samuels, W.D.; Exarhos, G.J. High-Dielectric-Constant Silver–Epoxy Composites as Embedded Dielectrics. Adv. Mater. 2005, 17, 1777–1781. [Google Scholar] [CrossRef]
- Shen, Y.; Lin, Y.; Li, M.; Nan, C.W. High Dielectric Performance of Polymer Composite Films Induced by a Percolating Interparticle Barrier Layer. Adv. Mater. 2007, 19, 1418–1422. [Google Scholar] [CrossRef]
- Xu, J.W.; Wong, C.P. Low-loss percolative dielectric composite. Appl. Phys. Lett. 2005, 87, 082907. [Google Scholar] [CrossRef]
- Sànchez-López, J.C.; Caballero, A.; Fernández, A. Characterisation of Passivated Aluminium Nanopowders: An XPS and TEM/EELS Study. J. Eur. Ceram. Soc. 1998, 18, 1195–1200. [Google Scholar] [CrossRef]
- Gromov, A.; Ilyin, A.; Förter-Barth, U.; Teipel, U. Characterization of Aluminum Powders: II. Aluminum Nanopowders Passivated by Non-inert Coatings. Propellants. Explos. Pyrotech. 2006, 31, 401–409. [Google Scholar] [CrossRef]
- Shahravan, A.; Desai, T.; Matsoukas, T. Passivation of Aluminum Nanoparticles by Plasma-Enhanced Chemical Vapor Deposition for Energetic Nanomaterials. ACS Appl. Mater. Interfaces 2014, 6, 7942–7947. [Google Scholar] [CrossRef] [PubMed]
- Jouet, R.J.; Warren, A.D.; Rosenberg, D.M.; Bellitto, V.J.; Park, K.; Zachariah, M.R. Surface Passivation of Bare Aluminum Nanoparticles Using Perfluoroalkyl Carboxylic Acids. Chem. Mater. 2005, 17, 2987–2996. [Google Scholar] [CrossRef]
- Jouet, R.J.; Carney, J.R.; Granholm, R.H.; Sandusky, H.W.; Warren, A.D. Preparation and Reactivity Analysis of Novel Perfluoroalkyl Coated Aluminium Nanocomposites. Mater. Sci. Technol. 2006, 22, 422–429. [Google Scholar] [CrossRef]
- Kappagantula, K.S.; Pantoya, M.L.; Horn, J. Effect of Surface Coatings on Aluminum Fuel Particles toward Nanocomposite Combustion. Surf. Coat. Technol. 2013, 237, 456–459. [Google Scholar] [CrossRef]
- Crouse, C.A.; Pierce, C.J.; Spowart, J.E. Influencing Solvent Miscibility and Aqueous Stability of Aluminum Nanoparticles through Surface Functionalization with Acrylic Monomers. ACS Appl. Mater. Interfaces 2010, 2, 2560–2569. [Google Scholar] [CrossRef]
- Guo, Z.; Pereira, T.; Choi, O.; Wang, Y.; Hahn, H.T. Surface functionalized alumina nanoparticle filled polymeric nanocomposites with enhanced mechanical properties. J. Mater. Chem. 2006, 16, 2800–2808. [Google Scholar] [CrossRef]
- Ash, B.J.; Rogers, D.F.; Weigand, C.J.; Schadler, L.S.; Siegel, R.W.; Benicewicz, B.C. Mechanical Properties of Alumina/Poly(methyl methacrylate) Nanocomposites. Applet. Polym. Compos. 2002, 23, 1014–1025. [Google Scholar] [CrossRef]
- Van Zyl, W.E.; García, M.; Schrauwen, B.A.G.; Kooi, B.J.; De Hosson, J.T.M.; Verweij, H. Hybrid Polyamide/Silica Nanocomposites: Synthesis and Mechanical Testing. Macromol. Mater. Eng. 2002, 287, 106–110. [Google Scholar] [CrossRef]
- Singha, S.; Thomas, M.J. Dielectric Properties of Epoxy Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 12–23. [Google Scholar] [CrossRef]
- Fischer, S.; Salcher, A.; Kornowski, A.; Weller, H.; Förster, S. Completely Miscible Nanocomposites. Angew. Chem. Int. Ed. 2011, 50, 7811–7814. [Google Scholar] [CrossRef] [PubMed]
- El-Shanshoury, A.I. Measurement of Dielectric Constant of Polystyrene, Potassium Chloride, and Gamma-Irradiated and Unirradiated Sodium Chloride Crystals at X-Band Microwave Frequencies. Arab J. Nucl. Sci. Appl. 2012, 45, 67–68. [Google Scholar]
- Fang, L.J.; Wu, W.; Huang, X.Y.; He, J.L.; Jiang, P.K. Hydrangea-like zinc oxide superstructures for ferroelectric polymer composites with high thermal conductivity and high dielectric constant. Compos. Sci. Technol. 2015, 107, 67–74. [Google Scholar] [CrossRef]
- Kim, K.; Park, M.S.; Na, Y.; Choi, J.; Jenekhe, S.A.; Kim, F.S. Preparation and application of polystyrene-grafted alumina core-shell nanoparticles for dielectric surface passivation in solution-processed polymer thin film transistors. Org. Electron. 2019, 65, 305–310. [Google Scholar] [CrossRef]
- Shen, Y.; Lin, Y.H.; Zhang, Q.M. Polymer Nanocomposites with High Energy Storage Densities. MRS Bull. 2015, 40, 753–759. [Google Scholar] [CrossRef]
- Kim, P.; Doss, N.M.; Tillotson, J.P.; Hotchkiss, P.J.; Pan, M.J.; Marder, S.R.; Li, J.Y.; Calame, J.P.; Perry, J.W. High Energy Density Nanocomposites Based on Surface-Modified BaTiO3 and a Ferroelectric Polymer. ACS Nano 2009, 3, 2581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hamidinejad, M.; Zhao, C.X.; Li, R.S.; Wang, S.; Kazemi, Y.; Park, C.B. A versatile foaming platform to fabricate polymer/carbon composites with high dielectric permittivity and ultra-low dielectric loss. J. Mater. Chem. A 2019, 7, 133–140. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Marian, C.; Liu, J.; Di, Q.; Xu, M.; Zhang, Y.; Han, W.; Liu, K. Polymer Grafted Aluminum Nanoparticles for Percolative Composite Films with Enhanced Compatibility. Polymers 2019, 11, 638. https://doi.org/10.3390/polym11040638
Yang C, Marian C, Liu J, Di Q, Xu M, Zhang Y, Han W, Liu K. Polymer Grafted Aluminum Nanoparticles for Percolative Composite Films with Enhanced Compatibility. Polymers. 2019; 11(4):638. https://doi.org/10.3390/polym11040638
Chicago/Turabian StyleYang, Chenggong, Chufarov Marian, Jie Liu, Qi Di, Mingze Xu, Yunhe Zhang, Wei Han, and Kun Liu. 2019. "Polymer Grafted Aluminum Nanoparticles for Percolative Composite Films with Enhanced Compatibility" Polymers 11, no. 4: 638. https://doi.org/10.3390/polym11040638
APA StyleYang, C., Marian, C., Liu, J., Di, Q., Xu, M., Zhang, Y., Han, W., & Liu, K. (2019). Polymer Grafted Aluminum Nanoparticles for Percolative Composite Films with Enhanced Compatibility. Polymers, 11(4), 638. https://doi.org/10.3390/polym11040638