Preparation and Characteristics of an Environmentally Friendly Hyperbranched Flame-Retardant Polyurethane Hybrid Containing Nitrogen, Phosphorus, and Silicon
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of DOPO-BQ–IPTS–TGIC
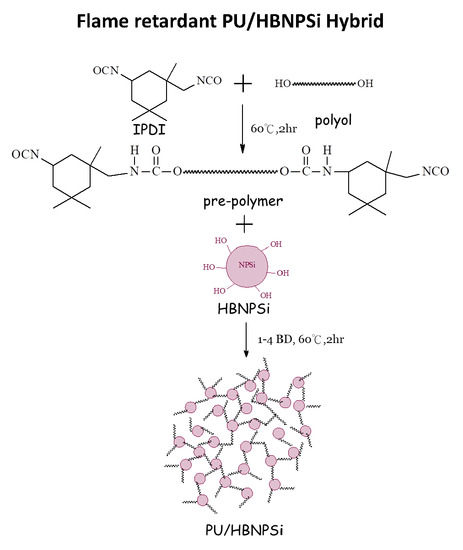
2.3. Preparation of PU/HBNPSi Hybrid
2.4. Measurements
3. Results and Discussion
3.1. Characterization of PU/HBNPSi Hybrid
3.2. Spectral Transmittance Analysis
3.3. Thermal Property
3.4. Integral Procedural Decomposition Temperature (IPDT)
3.5. TGΔ
3.6. Flame Retardancy of the Materials
3.7. Char Analysis by Raman Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heinen, M.; Gerbase, A.E.; Petzhold, C.L. Vegetable oil-based rigid polyurethanes and phosphorylated flame-retardants derived from epoxydized soybean oil. Polym. Degrad. Stab. 2014, 108, 76–86. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S. Synthesis and properties of rigid polyurethane foams synthesized from modified urea-formaldehyde resin. Constr. Build. Mater. 2019, 202, 718–726. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, Y.; Zhu, Y.; Xu, J.; Yao, Y. Preparation, characterization, and properties of novel ultraviolet-curable and flame-retardant polyurethane acrylate. Prog. Org. Coat. 2019, 129, 309–317. [Google Scholar] [CrossRef]
- Borreguero, M.A.; Sharma, P.; Spiteri, C.; Velencoso, M.M.; Carmona, S.M.; Moses, E.J.; Rodriguez, F.J. A novel click-chemistry approach to flame retardant polyurethanes. React. Funct. Polym. 2013, 73, 1207–1212. [Google Scholar] [CrossRef]
- Liu, X.; Guo, J.; Tang, W.; Li, H.; Gu, X.; Sun, J.; Zhang, S. Enhancing the flame retardancy of thermoplastic polyurethane by introducing montmorillonite nanosheets modified with phosphorylated chitosan. Compos. Part A 2019, 119, 291–298. [Google Scholar] [CrossRef]
- Mestry, S.; Kakatkar, R.; Mhaske, S.T. Cardanol derived P and Si based precursors to develop flame retardantPU coating. Prog. Org. Coat. 2019, 129, 59–68. [Google Scholar] [CrossRef]
- Qian, L.; Feng, F.; Tang, S. Bi-phase flame-retardant effect of hexa-phenoxy-cyclotriphosphazene on rigid polyurethane foams containing expandable graphite. Polymer 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Bing, H.; Lin, Y. Highly heat-resistant silicon-containing polyurethane-imide copolymers: Synthesis and thermal mechanical stability. Eur. Polym. J. 2017, 91, 337–353. [Google Scholar]
- Pagacz, J.; Hebdab, E.; Janowskic, B.; Sternikd, D.; Janciab, M.; Pielichowskib, K. Thermal decomposition studies on polyurethane elastomers reinforced with polyhedral silsesquioxanes by evolved gas analysis. Polym. Degrad. Stab. 2018, 149, 129–142. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Zhu, T.; Hua, Z.; Chen, Q.; Yu, X. Blends of thermoplastic polyurethane and polyether–polyimide: Preparation and properties. Polymer 2001, 42, 1493–1500. [Google Scholar] [CrossRef]
- Chen, X.; Ma, C.; Jiao, C. Enhancement of flame-retardant performance of thermoplastic polyurethane with the incorporation of aluminum hypophosphite and iron-graphene. Polym. Degrad. Stab. 2016, 129, 275–285. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, Q.; Zhang, N.; Guo, X.; Yan, H. Preparation of a novel polysiloxane and its synergistic effect with ammonium polyphosphate on the flame retardancy of polypropylene. Polym. Degrad. Stab. 2018, 150, 73–85. [Google Scholar] [CrossRef]
- Wen, J.Y.; Wilkes, G.L. Organic/Inorganic Hybrid Network Materials by the Sol-Gel Approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Morselli, D.; Bondioli, F.; Sangermano, M.; Messori, M. Photo-cured epoxy networks reinforced with TiO2 in-situ generated by means of non-hydrolytic sol-gel process. Polymer 2012, 53, 283–290. [Google Scholar] [CrossRef]
- Kusumoto, T.; Mori, Y.; Kanasaki, M.; Ueno, T.; Kameda, Y.; Oda, K.; Kodaira, S.; Kitamura, H.; Barillon, R.; Yamauchi, T. Yields on the formation of OH groups and the loss of CH groups along nuclear tracks in PADC films. Radiat. Meas. 2015, 83, 59–62. [Google Scholar] [CrossRef]
- Sritham, E.; Gunasekaran, S. FTIR spectroscopic evaluation of sucrose-maltodextrin-sodium citrate bioglass. Food Hydrocoll. 2017, 70, 371–382. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Xing, W.; Lu, H.; Hu, Y. An effective flame retardant for epoxy resins based on poly(DOPO substituted dihydroxyl phenyl pentaerythritol diphosphonate). Mater. Chem. Phys. 2011, 125, 536–541. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Hu, Y. Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym. Degrad. Stab. 2016, 133, 358–366. [Google Scholar] [CrossRef]
- Gallego, R.; Arteaga, J.F.; Valencia, C.; Franco, J.M. Thickening properties of several NCO-functionalized cellulose derivatives in castor oil. Chem. Eng. Sci. 2015, 134, 260–268. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Vouvoudi, E.C.; Papadopoulos, G.D. Epoxy polymer Hxtal NYL-1TM used in restoration and conservation: Irradiation with short and long wavelengths and study of photo-oxidation by FT-IR spectroscopy. J. Cult. Herit. 2016, 18, 279–289. [Google Scholar] [CrossRef]
- Bagherzadeh, M.R.; Daneshvar, A.; Shariatpanahi, H. Novel water-based nanosiloxane epoxy coating for corrosion protection of carbon steel. Surf. Coat. Technol. 2012, 206, 2057–2063. [Google Scholar] [CrossRef]
- Mahmoodian, M.; Arya, A.B.; Pourabbas, B. Synthesis of organic–inorganic hybrid compounds based on Bis-GMA and its sol-gel behavior analysis using Taguchi method. Dent. Mater. 2008, 24, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, M.; Przybylak, M.; Januszewski, R.; Maciejewski, H. Synthesis and flame retardant efficacy of hexakis(3-(triethoxysilyl)propyloxy)cyclotriphosphazene/silica coatings for cotton fabrics. Polym. Degrad. Stab. 2018, 148, 10–18. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Ramos, J.M.; Klein, R.; Lucas, A.D.; Rodriguez, F.J. Thermal degradation and fire behaviour of novel polyurethanes based on phosphate polyols. Polym. Degrad. Stab. 2014, 101, 40–51. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G. The novel silicon-containing epoxy/PEPA phosphate flame retardantfor transparent intumescent fire resistant coating. Appl. Surf. Sci. 2016, 385, 453–463. [Google Scholar] [CrossRef]
- Wu, C.S.; Liu, Y.L.; Chiu, Y.S. Epoxy resins possessing flame retardant elements from silicon incorporated epoxy compounds cured with phosphorus or nitrogen containing curing agents. Polymer 2002, 43, 4277–4284. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, L.; Ren, R.; Liu, S.; Chen, P. The thermal decomposition behavior and kinetics of epoxy resins cured with a novel phthalide-containing aromatic diamine. Polym. Test. 2018, 68, 46–52. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chen, S.; Min, Y.Q.; Qi, G.R. Synthesis of novel bisphenol containing phthalazinone and azomethine moieties and thermal properties of cured diamine/bisphenol/DGEBA polymers. Polymer 2006, 47, 1785–1795. [Google Scholar] [CrossRef]
- Hidalgo, J.; Fernández-Blázquez, J.P.; Jiménez-Morales, A.; Barriere, T.; Gelin, J.C.; Torralba, J.M. Effect of the particle size and solids volume fraction on the thermal degradation behaviour of Invar 36 feedstocks. Polym. Degrad. Stab. 2013, 98, 2546–2555. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Jiang, P.; Zhao, X.; Yu, H. Synthesis of a novel hybrid synergisticflame retardantand its application in PP/IFR. Polym. Degrad. Stab. 2011, 96, 1134–1140. [Google Scholar] [CrossRef]
- Gunasee, D.S.; Carrier, M.; Gorgens, F.J.; Mohee, R. Pyrolysis and combustion of municipal solid wastes: Evaluation of synergistic effects using TGA-MS. J. Anal. Appl. Pyrolysis 2016, 121, 50–61. [Google Scholar] [CrossRef]
- Kuan, C.F.; Yen, W.H.; Chen, C.H.; Yuen, S.M.; Kuan, H.C.; Chiang, C.L. Synthesis, characterization, flame retardance and thermal properties of halogen-free expandable graphite/PMMA composites prepared from sol-gel method. Polym. Degrad. Stab. 2008, 93, 1357–1363. [Google Scholar] [CrossRef]
- Yang, R.; Hu, W.; Xu, L.; Song, Y.; Li, J. Synthesis, mechanical properties and fire behaviors of rigid polyurethane foam with a reactive flame retardant containing phosphazene and phosphate. Polym. Degrad. Stab. 2015, 122, 102–109. [Google Scholar] [CrossRef]
- Pal, K.; Kang, D.J.; Kim, J.K. Microstructural investigations of zirconium oxide—On core-shell structure of carbon nanotubes. J. Nanopart. Res. 2011, 13, 2597–2607. [Google Scholar] [CrossRef]













| Sample No. | a Td10 (°C) | b Tmax (°C) | c Rmax (wt.%/min) | IPDT (°C) | d CY (wt.%) |
|---|---|---|---|---|---|
| Pristine PU | 302 | 343 | −36.0 | 348 | 0.7 |
| PU/HBNPSi 10% | 306 | 361 | −24.5 | 397 | 2.3 |
| PU/HBNPSi 20% | 308 | 365 | −21.7 | 416 | 3.8 |
| PU/HBNPSi 30% | 311 | 365 | −20.1 | 435 | 5.1 |
| PU/HBNPSi 40% | 326 | 376 | −17.0 | 488 | 8.1 |
| Sample | P% | Si% | LOI | ΔLOI | UL-94 |
|---|---|---|---|---|---|
| Pristine PU | 0 | 0 | 19 | 0 | Fail |
| PU/HBNPSi 10% | 0.35 | 0.39 | 21 | 2 | Fail |
| PU/HBNPSi 20% | 0.66 | 0.78 | 22 | 3 | Fail |
| PU/HBNPSi 30% | 0.98 | 1.33 | 23 | 4 | Fail |
| PU/HBNPSi 40% | 1.31 | 1.55 | 26 | 7 | V-2 |
| Sample No. | D-band | G-band | D/G | |
|---|---|---|---|---|
| 1350 cm−1 | 1580 cm−1 | |||
| PU/HBNPSi 10% | 1 min | 31,037 | 12,662 | 2.45 |
| 5 min | 26,999 | 23,826 | 1.33 | |
| PU/HBNPSi 40% | 1 min | 19,284 | 29,133 | 0.66 |
| 5 min | 15,703 | 39,396 | 0.40 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Chiang, C.-L. Preparation and Characteristics of an Environmentally Friendly Hyperbranched Flame-Retardant Polyurethane Hybrid Containing Nitrogen, Phosphorus, and Silicon. Polymers 2019, 11, 720. https://doi.org/10.3390/polym11040720
Chen C-H, Chiang C-L. Preparation and Characteristics of an Environmentally Friendly Hyperbranched Flame-Retardant Polyurethane Hybrid Containing Nitrogen, Phosphorus, and Silicon. Polymers. 2019; 11(4):720. https://doi.org/10.3390/polym11040720
Chicago/Turabian StyleChen, Chin-Hsing, and Chin-Lung Chiang. 2019. "Preparation and Characteristics of an Environmentally Friendly Hyperbranched Flame-Retardant Polyurethane Hybrid Containing Nitrogen, Phosphorus, and Silicon" Polymers 11, no. 4: 720. https://doi.org/10.3390/polym11040720
APA StyleChen, C. -H., & Chiang, C. -L. (2019). Preparation and Characteristics of an Environmentally Friendly Hyperbranched Flame-Retardant Polyurethane Hybrid Containing Nitrogen, Phosphorus, and Silicon. Polymers, 11(4), 720. https://doi.org/10.3390/polym11040720