Preparation and Characterization of Whey Protein-Based Polymers Produced from Residual Dairy Streams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
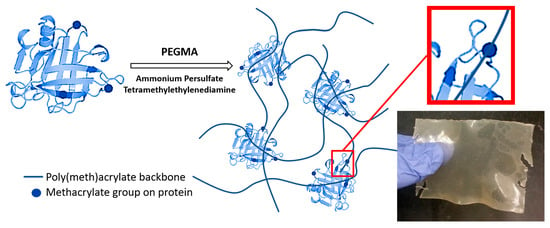
2.2. Methacrylation and Polymerization Reactions
2.3. Mechanical and Thermal Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Protein/PEGMA Ratio
3.2. Effect of Protein in Water Concentrations
3.3. Thermal Stability Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cuq, B.; Gontard, N.; Guilbert, S. Proteins as Agricultural Polymers for Packaging Production. Cereal Chem. 1998, 75, 1–9. [Google Scholar] [CrossRef]
- Flieger, M.; Kantorová, M.; Prell, A.; Řezanka, T.; Votruba, J. Biodegradable plastics from renewable sources. Folia Microbiol. 2003, 48, 27. [Google Scholar] [CrossRef]
- Ceballos, L.S.; Morales, E.R.; de la Torre Adarve, G.; Castro, J.D.; Martínez, L.P.; Sampelayo, M.R.S. Composition of goat and cow milk produced under similar conditions and analyzed by identical methodology. J. Food Compos. Anal. 2009, 22, 322–329. [Google Scholar] [CrossRef]
- Brans, G.; Schroën, C.G.P.H.; van der Sman, R.G.M.; Boom, R.M. Membrane fractionation of milk: State of the art and challenges. J. Membr. Sci. 2004, 243, 263–272. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Marshall, K. Review: Therapeutic applications of whey protein. Altern. Med. Rev. 2004, 9, 136–157. [Google Scholar] [PubMed]
- Kosikowski, F.V. Whey utilization and whey products. J. Dairy Sci. 1979, 62, 1149–1160. [Google Scholar] [CrossRef]
- Adamson, N. Chapter 15 Whey processing. In Dairy Processing Handbook; Pak, T., Ed.; Tetra Pak Processing Systems: Lausanne, Switzerland, 2003. [Google Scholar]
- Illanes, A. Whey upgrading by enzyme biocatalysis. Electron. J. Biotechnol. 2011, 14, 9. [Google Scholar] [CrossRef]
- Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol. Adv. 2010, 28, 375–384. [Google Scholar] [CrossRef]
- Siso, M.I.G. The biotechnological utilization of cheese whey: A review. Bioresour. Technol. 1996, 57, 1–11. [Google Scholar] [CrossRef]
- Mawson, A.J. Bioconversions for whey utilization and waste abatement. Bioresour. Technol. 1994, 47, 195–203. [Google Scholar] [CrossRef]
- ADPI. 2012 Dairy Products Utilization & Production Trends; American Dairy Products Institute: Elmhurst, IL, USA, 2013. [Google Scholar]
- Saad, S.; Dairy Waste in Al Rawabi Dairy Company (Al Rawabi Dairy Company, Dubai, UAE). Personal communication, 2017.
- Khwaldia, K.; Perez, C.; Banon, S.; Desobry, S.; Hardy, J. Milk Proteins for Edible Films and Coatings. Crit. Rev. Food Sci. Nutr. 2004, 44, 239–251. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Wagh, Y.R.; Pushpadass, H.A.; Emerald, F.M.; Nath, B.S. Preparation and characterization of milk protein films and their application for packaging of Cheddar cheese. J. Food Sci. Technol. 2014, 51, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Mandal, A.; Sharma, S. Protein-based bioplastics and their antibacterial potential. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Swain, S.N.; Biswal, S.M.; Nanda, P.K.; Nayak, P.L. Biodegradable Soy-Based Plastics: Opportunities and Challenges. J. Polym. Environ. 2004, 12, 35–42. [Google Scholar] [CrossRef]
- Salmoral, E.M.; Gonzalez, M.E.; Mariscal, M.P.; Medina, L.F. Comparison of chickpea and soy protein isolate and whole flour as biodegradable plastics. Ind. Crops Prod. 2000, 11, 227–236. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.B.; Nichol, J.W.; Aubin, H.; Bae, H.; Yamanlar, S.; Al-Haque, S.; Koshy, S.T.; Khademhosseini, A. Synthesis and characterization of tunable poly(ethylene glycol): Gelatin methacrylate composite hydrogels. Tissue Eng. Part A 2011, 17, 1713–1723. [Google Scholar] [CrossRef]
- Bochenski, T. Techno-Economic Analysis of Microalgae Biorefinery in Arid Environments for Co-Production of Biofuels and Value-Added Compounds. Ph.D. Thesis, Masdar Campus, Khalifa University, Abu Dhabi, UAE, 2017. [Google Scholar]
- Chan, W.Y.; Bochenski, T.; Schmidt, J.E.; Olsen, B.D. Peptide Domains as Reinforcement in Protein-Based Elastomers. ACS Sustain. Chem. Eng. 2017, 5, 8568–8578. [Google Scholar] [CrossRef]
- Banker, G.S. Film coating theory and practice. J. Pharm. Sci. 1966, 55, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Morr, C.V.; Ha, E.Y. Whey protein concentrates and isolates: Processing and functional properties. Crit. Rev. Food Sci. Nutr. 1993, 33, 431–476. [Google Scholar] [CrossRef]
- Kadam, D.M.; Thunga, M.; Wang, S.; Kessler, M.R.; Grewell, D.; Lamsal, B.; Yu, C. Preparation and characterization of whey protein isolate films reinforced with porous silica coated titania nanoparticles. J. Food Eng. 2013, 117, 133–140. [Google Scholar] [CrossRef]
- MatWeb. Overview of Materials for Low Density Polyethylene (LDPE), Extrusion Grade. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=8e92b9e66e214e1b8fc8222dbff6d480 (accessed on 12 August 2018).
- MatWeb. Overview of Materials for High Density Polyethylene (HDPE), Extruded. Available online: http://www.matweb.com/search/datasheet.aspx?matguid=482765fad3b443169ec28fb6f9606660&ckck=1 (accessed on 12 August 2018).
- Rudnik, E. Chapter 4—Thermal and thermooxidative degradation. In Compostable Polymer Materials; Rudnik, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 72–85. [Google Scholar]
- Kayacan, İ.; Doğan, Ö.M. Pyrolysis of Low and High Density Polyethylene. Part I: Non-isothermal Pyrolysis Kinetics. Energy Source Part A 2008, 30, 385–391. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalermthai, B.; Chan, W.Y.; Bastidas-Oyanedel, J.-R.; Taher, H.; Olsen, B.D.; Schmidt, J.E. Preparation and Characterization of Whey Protein-Based Polymers Produced from Residual Dairy Streams. Polymers 2019, 11, 722. https://doi.org/10.3390/polym11040722
Chalermthai B, Chan WY, Bastidas-Oyanedel J-R, Taher H, Olsen BD, Schmidt JE. Preparation and Characterization of Whey Protein-Based Polymers Produced from Residual Dairy Streams. Polymers. 2019; 11(4):722. https://doi.org/10.3390/polym11040722
Chicago/Turabian StyleChalermthai, Bushra, Wui Yarn Chan, Juan-Rodrigo Bastidas-Oyanedel, Hanifa Taher, Bradley D. Olsen, and Jens Ejbye Schmidt. 2019. "Preparation and Characterization of Whey Protein-Based Polymers Produced from Residual Dairy Streams" Polymers 11, no. 4: 722. https://doi.org/10.3390/polym11040722
APA StyleChalermthai, B., Chan, W. Y., Bastidas-Oyanedel, J. -R., Taher, H., Olsen, B. D., & Schmidt, J. E. (2019). Preparation and Characterization of Whey Protein-Based Polymers Produced from Residual Dairy Streams. Polymers, 11(4), 722. https://doi.org/10.3390/polym11040722