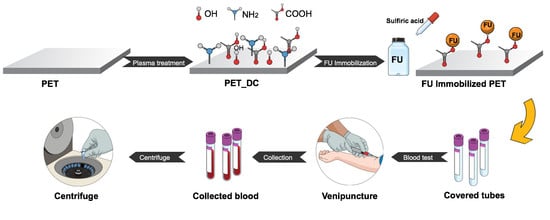
Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Anticoagulant Surfaces
2.2. Surface Wettability Evaluation
2.3. Surface Morphology Investigation by SEM
2.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR) Analysis
2.5. X-Ray Photoelectron Spectroscopy (XPS) Analysis
2.6. Evaluation of Anticoagulation Activity
3. Results
3.1. Surface Wettability Behavior
3.2. Surface Morphology
3.3. Surface Chemical Anaylsis by ATR-FTIR
3.4. Surface Elemental Analysis by XPS
3.5. Blood Coagulation Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mozetic, M.; Ostrikov, K.; Ruzic, D.N.; Curreli, D.; Cvelbar, U.; Vesel, A.; Primc, G.; Leisch, M.; Jousten, K.; Malyshev, O.B.; et al. Recent advances in vacuum sciences and applications. J. Phys. D Appl. Phys. 2014, 47, 153001:1–153001:23. [Google Scholar] [CrossRef]
- Mozetic, M.; Primc, G.; Vesel, A.; Zaplotnik, R.; Modic, M.; Junkar, I.; Recek, N.; Klanjsek-Gunde, M.; Guhy, L.; Sunkara, M.K.; et al. Application of extremely non-equilibrium plasmas in the processing of nano and biomedical materials. Plasma Sources Sci. Technol. 2015, 24, 015026:1–015026:12. [Google Scholar] [CrossRef]
- Mozetic, M.; Vesel, A.; Primc, G.; Eisenmenger-Sittner, C.; Bauer, J.; Eder, A.; Schmid, G.H.S.; Ruzic, D.N.; Ahmed, Z.; Barker, D.; et al. Recent developments in surface science and engineering, thin films, nanoscience, biomaterials, plasma science, and vacuum technology. Thin Solid Films 2018, 660, 120–160. [Google Scholar] [CrossRef]
- Hasebe, T.; Nagashima, S.; Kamijo, A.; Moon, M.W.; Kashiwagi, Y.; Hotta, A.; Lee, K.R.; Takahashi, K.; Yamagami, T.; Suzuki, T. Hydrophobicity and non-thrombogenicity of nanoscale dual rough surface coated with fluorine-incorporated diamond-like carbon films: Biomimetic surface for blood-contacting medical devices. Diam. Relat. Mater. 2015, 38, 14–18. [Google Scholar] [CrossRef]
- Reviakine, I.; Jung, F.; Braune, S.; Brash, J.L.; Latour, R.; Gorbet, M.; Oeveren, W.V. Stirred, shaken, or stagnant: What goes on at the blood–biomaterial interface. Blood Rev. 2017, 31, 11–21. [Google Scholar] [CrossRef]
- Alibeik, S.; Zhu, S.; Yau, J.W.; Weitz, J.I.; Brash, J.L. Surface modification with polyethylene glycol–corn trypsin inhibitor conjugate to inhibit the contact factor pathway on blood-contacting surfaces. Acta Biomater. 2011, 7, 4177–4186. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Maitz, M.F.; Huang, N. Surface modification of cardiovascular materials and implants. Surf. Coat. Technol. 2013, 233, 80–90. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuang, G.; Zong, S.; Liu, S.; Xiao, H.; Chen, X.; Zhou, D.; Huang, Y. Sandwich-Like Fibers/Sponge Composite Combining Chemotherapy and Hemostasis for Efficient Postoperative Prevention of Tumor Recurrence and Metastasis. Adv. Mater. 2018, 30, 1803217. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Yamazoe, H.; Oyane, A.; Nashima, T.; Ito, A. Reduced platelet adhesion and blood coagulation on cross-linked albumin films. Mater. Sci. Eng. C 2010, 30, 812–816. [Google Scholar] [CrossRef]
- Biran, R.; Pond, D. Heparin coatings for improving blood compatibility of medical devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef]
- Tengvall, P. Protein Interactions with Biomaterials. In Comprehensive Biomaterials, 1st ed.; Hutmacher, D.W., Grainger, D.W., Ducheyne, P., Eds.; Elsevier Ltd.: Philadelphia, PA, USA, 2011; Volume 4, pp. 63–73. [Google Scholar]
- Horbett, T.A. Adsorbed proteins on biomaterials. In Biomaterials Science, an Introduction to Materials in Medicine, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Inc.: Oxford, UK, 2013; pp. 394–408. [Google Scholar]
- Xu, L.C.; Siedlecki, C.A. Heparin coatings for improving blood compatibility of medical devices. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface Modifications for Antifouling Membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Seyfert, U.T.; Biehl, V.; Schenk, J. In vitro hemocompatibility testing of biomaterials according to the ISO 10993-4. Biomol. Eng. 2002, 19, 91–96. [Google Scholar] [CrossRef]
- Xu, L.C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B 2014, 124, 49–68. [Google Scholar] [CrossRef]
- Courtney, J.M.; Lamba, N.M.K.; Sundaram, S.; Forbes, C.D. Biomaterials for blood-contacting applications. Biomaterials 1994, 15, 737–744. [Google Scholar] [CrossRef]
- Faxalv, L.; Ekblad, T.; Liedberg, B.; Lindahl, T.L. Blood compatibility of photografted hydrogel coatings. Acta Biomater. 2010, 6, 2599–2608. [Google Scholar] [CrossRef]
- Cashman, J.D.; Kennah, E.; Shuto, A.; Winternitz, C.; Springate, C.M.K. Fucoidan film safely inhibits surgical adhesions in a rat model. J. Surg. Res. 2011, 171, 495–503. [Google Scholar] [CrossRef]
- Ikada, Y. Surface modification of polymers for medical applications. Biomaterials 1994, 15, 725–736. [Google Scholar] [CrossRef]
- Hsiao, C.R.; Lin, C.W.; Chou, C.M.; Chung, C.J.; He, J.L. Surface modification of blood-contacting biomaterials by plasma-polymerized superhydrophobic films using hexamethyldisiloxane and tetrafluoromethane as precursors. Appl. Surf. Sci. 2015, 346, 50–56. [Google Scholar] [CrossRef]
- Lehocky, M.; Amaral, P.F.F.; Coelho, M.A.Z.; Stahel, P.; Barros-Timmons, A.M.; Coutinho, J.A.P. Attachment/detachment of Saccharomyces cerevisiae on plasma deposited organosilicon thin films. Czechoslov. J. Phys. 2006, 56, 1256–1262. [Google Scholar] [CrossRef]
- Lehocky, M.; Lapcik, L.; Dlabaja, R.; Rachunek, L. Influence of artificially accelerated ageing on the adhesive joint of plasma treated polymer materials. Czechoslov. J. Phys. 2004, 54, C533–C538. [Google Scholar]
- Lehocky, M.; Amaral, P.F.F.; Stahel, P.; Coelho, M.A.Z.; Barros-Timmons, A.M.; Coutinho, J.A.P. Preparation and characterization of organosilicon thin films for selective adhesion of Yarrowia lipolytica yeast cells. J. Chem. Technol. 2007, 82, 360–366. [Google Scholar]
- Lehocky, M.; Stahel, P.; Koutny, M.; Cech, J.; Institoris, J.; Mracek, A. Adhesion of Rhodococcus sp. S3E2 and Rhodococcus sp. S3E3 to plasma prepared Teflon-like and organosilicon surfaces. J. Mater. Process. Technol. 2009, 209, 2871–2875. [Google Scholar] [CrossRef]
- Goa, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar]
- Ding, Q.; Xu, X.; Yue, Y.; Mei, C.; Huang, C.; Jiang, S.; Wu, X.; Han, J. Nanocellulose-Mediated Electroconductive Self-Healing Hydrogels with High Strength, Plasticity, Viscoelasticity, Stretchability, and Biocompatibility toward Multifunctional Applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Fu, Q.; Ma, Z.; Fang, P.; He, C. Microstructure and surface state of plasma-treated high-density polyethylene elucidated by energy-tunable positron annihilation and water contact angle measurements. JJAP Conf. Proc. 2014, 2, 011202. [Google Scholar] [CrossRef]
- Azevedo, T.C.G.; Bezerra, M.E.B.; Santos, M.D.G.D.L.; Souza, L.A.; Marques, C.T.; Benevides, N.M.B.; Leite, E.L. Heparinoids algal and their anticoagulant, hemorrhagic activities and platelet aggregation. Biomed. Pharmacother. 2009, 63, 477–483. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Alves, M.G.C.F.; Will, L.S.E.P.; Costa, T.G.; Sabry, D.A.; Rego, L.A.R.S.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef]
- Hu, Y.; Li, S.; Li, J.; Ye, X.; Ding, T.; Liu, D.; Chen, J.; Ge, Z.; Chen, S. Identification of a highly sulfated fucoidan from sea cucumber Pearsonothuria graeffei with well-repeated tetrasaccharides units. Carbohydr. Polym. 2015, 134, 808–816. [Google Scholar] [CrossRef]
- Mracek, A.; Varhanikova, J.; Lehocky, M.; Grundelova, L.; Pokopcova, A.; Velebny, V. The influence of hofmeister series ions on hyaluronan swelling and viscosity. Molecules 2008, 13, 1025–1034. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Gerbst, A.G.; Ushakova, N.A.; Tsvetkova, E.A.; Dmitrenok, A.S.; Usov, A.I.; Nifantiev, N.E. Anticoagulant and antithrombotic activities of modified xylofucan sulfate from the brown alga Punctaria plantaginea. Carbohydr. Polym. 2016, 136, 826–833. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Q.; Chen, L.; Ren, S.; Xu, P.; Tang, Y. Higher specificity of the activity of low molecular weight fucoidan for thrombin-induced platelet aggregation. Thromb. Res. 2010, 125, 419–426. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from Fucoidan; Multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Liewert, I.; Ehrig, K.; Alban, S. Effects of fucoidans and heparin on reactions of neutrophils induced by IL-8 and C5a. Carbohydr. Polym. 2017, 165, 462–469. [Google Scholar] [CrossRef]
- Kim, J.M.; Bae, I.H.; Lima, K.S.; Park, J.K.; Park, D.S.; Lee, S.Y.; Jang, E.J.; Ji, M.S.; Sim, D.S.; Hong, Y.J.; et al. A method for coating fucoidan onto bare metal stent and in vivo evaluation. Prog. Org. Coat. 2015, 78, 348–356. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, R.; Li, M.; Liang, X.; Elmada, Z.C. Effects of dietary fucoidan on the blood constituents, anti-oxidation and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Fish Shelfish Immun. 2014, 41, 264–270. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Pielesz, A.; Binias, W. Cellulose acetate membrane electrophoresis and FTIR spectroscopy as methods of identifying a fucoidan in Fucus vesiculosus Linnaeus. Carbohydr. Res. 2010, 345, 2676–2682. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, S.; Wang, J.; Li, F.; Chen, A.; Li, B. A comparative study of antithrombotic and antiplatelet activities of different fucoidans from Laminaria japonica. Thromb. Res. 2012, 129, 771–778. [Google Scholar] [CrossRef]
- Tengdelius, M.; Lee, C.J.; Grenegard, M.; Griffith, M.; Pahlsson, P.; Konradsson, P. Synthesis and biological evaluation of fucoidan-mimetic glycopolymers through cyanoxyl-mediated free-radical polymerization. Biomacromolecules 2014, 15, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Rabanal, M.; Ponce, N.M.; Navarro, D.; Gomez, R.M.; Stortz, C. The system of fucoidans from the brown seaweed Dictyota dichotoma: Chemical analysis and antiviral activity. Carbohydr. Polym. 2014, 101, 804–811. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A.; Mozetic, M.; Strnad, S. Improvement of adhesion of fucoidan on polyethylene terephthalate surface using gas plasma treatments. Vacuum 2011, 85, 1083–1086. [Google Scholar] [CrossRef]
- Ozaltin, K.; Lehocky, M.; Humpolicek, P.; Pelkova, J.; Saha, P. A new route of fucoidan immobilization on low density polyethylene and its blood compatibility and anticoagulation activity. Int. J. Mol. Sci. 2016, 17, 908. [Google Scholar] [CrossRef] [PubMed]
- Mracek, A.; Lehocky, M.; Smolka, P.; Grulich, O.; Velebny, V. The allyamine grafting on the plasma pre-treated polyester nonwowen fabric: Preperation, characterization and utilization. Fiber Polym. 2010, 11, 1106–1110. [Google Scholar] [CrossRef]
- Almazan, M.C.A.; Paredes, J.I.; Mendoza, M.P.; Garcia, M.D.; Garzon, F.J.L.; Alonso, A.M.; Tascon, J.M.D. Surface characterisation of plasma-modified poly(ethylene terephthalate). J. Colloid Interface Sci. 2006, 293, 353–363. [Google Scholar] [CrossRef]
- Andanson, J.M.; Kazarian, S.G. In situ ATR-FTIR spectroscopy of poly(ethylene terephthalate) subjected to high-temperature methanol. Macromol. Symp. 2008, 265, 195–204. [Google Scholar] [CrossRef]
- Huang, L.Y.; Yang, M.C. Surface immobilization of chondroitin 6-sulfate/heparin multilayer on stainless steel for developing drug-eluting coronary stents. Colloids Surf. B 2008, 61, 43–52. [Google Scholar] [CrossRef]
- Humpolicek, P.; Kucekova, Z.; Kasparkova, V.; Pelkova, J.; Modic, M.; Junkar, I.; Trchova, M.; Bober, P.; Stejskal, J.; Lehocky, M. Blood coagulation and platelet adhesion on polyaniline films. Colloids Surf. B 2015, 133, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, W.A.; Jeon, Y.J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Harris, L.F.; Lopez, V.C.; Killard, A.J. Coagulation monitoring devices: Past, present, and future at the point of care. Trends Anal. Chem. 2013, 50, 85–95. [Google Scholar] [CrossRef] [Green Version]





| Samples | Contact Angle (°) |
|---|---|
| PET | 70.6 ± 0.60 |
| PET_DC | 22.09 ± 1.32 |
| FU3 | 43.21 ± 2.67 |
| FU4 | 39.08 ± 1.74 |
| FU5 | 42.61 ± 2.92 |
| FU6 | 39.74 ± 5.52 |
| FU7 | 42.54 ± 7.45 |
| Samples | C1s | O1s | N1s | S2p | O1s/C1s | N1s/C1s | S2p/C1s |
|---|---|---|---|---|---|---|---|
| PET | 69.7 | 30.3 | 0 | 0 | 0.435 | 0 | 0 |
| PET_DC | 58.4 | 40.9 | 0.7 | 0 | 0.700 | 0.012 | 0 |
| FU3 | 63.4 | 35.7 | 0 | 0.2 | 0.563 | 0 | 0.003 |
| FU4 | 63.7 | 34.5 | 0.2 | 1.6 | 0.542 | 0.003 | 0.025 |
| FU5 | 63.6 | 34.4 | 0.2 | 1.8 | 0.541 | 0.003 | 0.028 |
| FU6 | 63.7 | 35.7 | 0.1 | 0.5 | 0.560 | 0.002 | 0.008 |
| FU7 | 63.8 | 35.6 | 0.1 | 0.5 | 0.558 | 0.002 | 0.008 |
| Samples | PT | aPTT | TT |
|---|---|---|---|
| PET | 12.6 | 26.3 | 15.5 |
| PET_DC | 10.9 | 22 | 15.2 |
| FU3 | 11.3 | 29.4 | 34.7 |
| FU4 | 11 | 35.1 | 88.7 |
| FU5 | 10.9 | 35.1 | 100+ |
| FU6 | 11.1 | 30.8 | 58.6 |
| FU7 | 11.2 | 31.5 | 60.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozaltin, K.; Lehocky, M.; Humpolicek, P.; Pelkova, J.; Di Martino, A.; Karakurt, I.; Saha, P. Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization. Polymers 2019, 11, 750. https://doi.org/10.3390/polym11050750
Ozaltin K, Lehocky M, Humpolicek P, Pelkova J, Di Martino A, Karakurt I, Saha P. Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization. Polymers. 2019; 11(5):750. https://doi.org/10.3390/polym11050750
Chicago/Turabian StyleOzaltin, Kadir, Marian Lehocky, Petr Humpolicek, Jana Pelkova, Antonio Di Martino, Ilkay Karakurt, and Petr Saha. 2019. "Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization" Polymers 11, no. 5: 750. https://doi.org/10.3390/polym11050750
APA StyleOzaltin, K., Lehocky, M., Humpolicek, P., Pelkova, J., Di Martino, A., Karakurt, I., & Saha, P. (2019). Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization. Polymers, 11(5), 750. https://doi.org/10.3390/polym11050750