Effects of Gas Adsorption on the Mechanical Properties of Amorphous Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
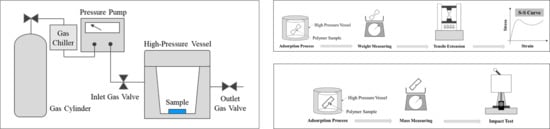
2.2. Method
2.2.1. Experiment on Change in Impact Strength
2.2.2. Experiment on Change in Tensile Strength
2.3. Experimental Conditions
2.3.1. Experiment on Change in Impact Strength
2.3.2. Experiment on Change in Tensile Strength
3. Results and Discussion
3.1. Changes in Impact Strength by CO2 Adsorption
3.2. Changes in Tensile Strength by CO2 Adsorption
4. Conclusions
4.1. Impact Strength Change through CO2 Adsorption
- Based on an observation of the sorption rate and volume expansion, the free volume can be confirmed through the part with sorption rate that does not cause a change in volume, and the volume expansion is linearly related to the sorption rate in the part having a sorption rate of 2% or higher.
- It was also confirmed that the CO2 improves the impact strength of the APET by volume increase, by acting as a cushion. Also, the plasticizing effect of CO2 works on the sample, even in a solid-state polymer sample. The rapid increase in the impact strength is equal to a sorption rate of 2%, which is the starting point for the volumetric expansion. A maximum value 956% higher than that of the reference specimen at a sorption rate of 2.85% was observed.
- It was confirmed that CO2 dissolved in the polymer has the effect of increasing the impact strength in a specific sorption rate.
4.2. Tensile Strength Change through CO2 Adsorption
- As the amount of dissolved CO2 increases, the tensile strength decreases. In this experiment, the sorption rate was changed by varying the exposure time (sorption time) to high-pressure CO2, and the tensile strength was decreased with this change.
- The amount of CO2 dissolution affects not only the tensile strength but also the deformed stress-strain curve formation of APET. In particular, when CO2 was dissolved over a certain degree of sorption rate (6.67%), a homogenous deformation occurred without the occurrence of necking on the stress–strain curve.
- The amount of dissolved CO2 and the tensile strength have a linear correlation (decrease in tensile strength ∝ sorption rate), and the extent of reduction in the tensile strength increases as the amount of dissolved CO2 increases.
- Changes in the mechanical properties from CO2 dissolution are related to the network density of the amorphous region of the polymeric materials. As the amount of dissolved gas increases, the increase in area and volume increases. In addition, the sorption rate and volume of the gas decrease again as the retention time (desorption time) increases after sample removal from the high-pressure condition, and thus the tensile strength is also close to the value of the reference specimen.
Author Contributions
Funding
Conflicts of Interest
References
- Cha, S.W. A Microcellular Foaming/Forming Process Performed at Ambient Temperature and a Supermicrocellular Foaming Process. Ph.D. Thesis, Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA, 1994. [Google Scholar]
- Baldwin, D.F. Microcellular Polymer Processing and the Design of a Continuous Sheet Processing System. Ph.D. Thesis, Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA, 1994. [Google Scholar]
- Cha, S.W.; Yun, J.D. Change of Glass Transition Temperature of PETG Containing Gas. Korean Soc. Mech. Eng. 2000, 24, 824–829. [Google Scholar]
- Arun, P.; Gregory, W.; Vipin, K.; Mark, T.; Karl, S. Effect of CO2 Sorption and Desorption on the Creep Response of Polycarbonate. Polym. Eng. Sci. 2005, 45, 1639–1644. [Google Scholar]
- Fraunhofer UMSICHT studies customization of plastics via CO2 impregnation. Addit. Polym. 2011, 2011, 9.
- Kim, H.B. The Effect of Gas Absorption Induced a Change of Solubility in Microcellular Foamed Process; Yonsei University: Seoul, Korea, 2005. [Google Scholar]
- Vipin, K.; Michael, V.; John, W.; Karl, S. Experimental Characterization of the Tensile Behavior of Microcellular Polycarbonate Foams. J. Eng. Mater. Technol. 1994, 116, 439–445. [Google Scholar]
- Sauer, J.A. Deformation, Yield and Fracture of Polymers at High Pressure. Polym. Eng. Sci. 1977, 17, 150–164. [Google Scholar] [CrossRef]
- Guoming, L.; Liuchun, Z.; Xiuqin, Z.; Chuncheng, L.; Dujin, W. Stress induced lamellar thickening in poly(ethylene succinate). Polymer 2013, 54, 6860–6866. [Google Scholar]
- Lee, J.G.; Park, S.H.; Kim, S.H. Investigation of Properties of the PET Film Dependent on the Biaxial Stretching. Polymer 2010, 34, 579–587. [Google Scholar]
- Park, C.B. The Role of Polymer/Gas Solution in Continuous Processing of Microcellular Polymers. Ph.D. Thesis, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA, 1993. [Google Scholar]
- Mashmood, S.H. Dimethyl ether’s plasticizing effect on carbon dioxide solubility in polystyrene. Polymer 2016, 97, 95–103. [Google Scholar] [CrossRef]
- Mashmood, S.H. Study of volume swelling and interfacial tension of the polystyrene–carbon dioxide–dimethyl ether system. Polymer 2015, 456, 174–181. [Google Scholar]
- Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics; ASTM Standard D256; ASTM International: West Conshohocken, PA, USA, 2016.
- Standard Test Method for Tensile Properties of Plastics; ASTM Standard D638; ASTM International: West Conshohocken, PA, USA, 2016.
- Da, L.; Tao, L.; Ling, Z.; Wei, Y. Solubility and Diffusivity of Carbon Dioxide in Solid-State Isotactic Polypropylene by the Pressure-Decay Method. Ind. Eng. Chem. Res. 2009, 48, 7117–7124. [Google Scholar]
- Van Melick, H.G.H.; Govaert, L.E.; Meijer, H.E.H. On the origin of strain hardening in glassy polymers. Polymer 2003, 44, 2493–2502. [Google Scholar] [CrossRef] [Green Version]
- Ronald, P. White Polymer Free Volume and Its Connection to the Glass Transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar]
- Li, X.K.; Lu, H.; Cao, G.P.; Qian, Y.H.; Chen, L.H.; Zhang, R.H.; Liu, H.L.; Shi, Y.H. Experimental Study of the Synergistic Plasticizing Effect of Carbon Dioxide and Ibuprofen on the Glass Transition Temperature of Poly(methyl methacrylate). Ind. Eng. Chem. Res. 2014, 53, 5873–5885. [Google Scholar] [CrossRef]














| Material | APET | |
|---|---|---|
| Adsorption Condition | Gas Pressure (MPa) | 5 |
| Vessel Temperature (°C) | 23 | |
| Adsorption Time (h) | 1–120 | |
| Desorption Condition | Desorption Pressure (MPa) | 0.1 |
| Desorption Temperature (°C) | 23 | |
| Desorption Time (min) | 4 | |
| Material | APET | |
|---|---|---|
| Adsorption Condition | Saturation Pressure (MPa) | 6 |
| Saturation Temperature (°C) | 23 | |
| Saturation Time (h) | 4/8/12/16 | |
| Desorption Condition | Desorption Pressure (MPa) | 0.1 |
| Desorption Temperature (°C) | 23 | |
| Desorption Time (min) | 4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.W.; Sohn, J.S.; Kim, H.K.; Ryu, Y.; Cha, S.W. Effects of Gas Adsorption on the Mechanical Properties of Amorphous Polymer. Polymers 2019, 11, 817. https://doi.org/10.3390/polym11050817
Kim SW, Sohn JS, Kim HK, Ryu Y, Cha SW. Effects of Gas Adsorption on the Mechanical Properties of Amorphous Polymer. Polymers. 2019; 11(5):817. https://doi.org/10.3390/polym11050817
Chicago/Turabian StyleKim, Shin Won, Joo Seong Sohn, Hyun Keun Kim, Youngjae Ryu, and Sung Woon Cha. 2019. "Effects of Gas Adsorption on the Mechanical Properties of Amorphous Polymer" Polymers 11, no. 5: 817. https://doi.org/10.3390/polym11050817
APA StyleKim, S. W., Sohn, J. S., Kim, H. K., Ryu, Y., & Cha, S. W. (2019). Effects of Gas Adsorption on the Mechanical Properties of Amorphous Polymer. Polymers, 11(5), 817. https://doi.org/10.3390/polym11050817