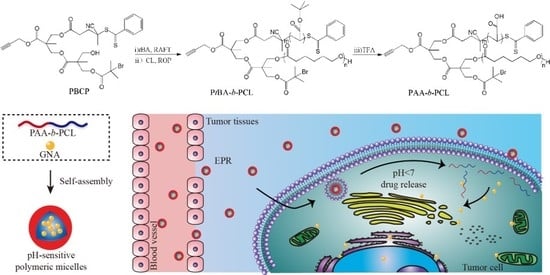
Amphiphilic Block Copolymer Poly (Acrylic Acid)-B-Polycaprolactone as a Novel pH-sensitive Nanocarrier for Anti-Cancer Drugs Delivery: In-vitro and In-vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PAA-b-PCL
2.3. Formation of Blank and GNA-Loaded PMs
2.4. pH-Triggered Reassembly of PMs and GNA-Loaded PMs Releasing Behavior
2.5. Cell Viability Assay (MTT)
2.6. Cellular Uptake
2.7. In Vivo Studies of GNA-Loaded PMs
2.8. Statistical Analysis
2.9. Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of PAA-b-PCL
3.2. Formation and Stimuli-Triggered Morphological Transition of PMs
3.3. DLC, DLE and In Vitro Releasing Behavior
3.4. Cytotoxicity and In Vitro Cellular Uptake Assays
3.5. Pharmacokinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, H.; Su, J.J.; Peng, J.Y.; Wang, M.; Wang, X.C.; Yan, F.G.; Wang, X.S.; Li, Q.L. Gambogenic acid inhibits proliferation of a549 cells through apoptosis inducing through up-regulation of the p38 mapk cascade. J. Asian Nat. Prod. Res. 2011, 13, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Cheng, H.; Zhu, G.Q.; Yang, L.; Zhou, A.; Wang, X.; Fang, N.; Xia, L.; Su, J.; Wang, M.; et al. Gambogenic acid inhibits proliferation of a549 cells through apoptosis-inducing and cell cycle arresting. Biol. Pharm. Bull. 2010, 33, 415–420. [Google Scholar] [CrossRef]
- Yan, F.G.; Wang, M.; Li, J.M.; Cheng, H.; Su, J.; Wang, X.; Wu, H.; Xia, L.; Li, X.; Chang, H.C.; et al. Gambogenic acid induced mitochondrial-dependent apoptosis and referred to phospho-Erk1/2 and phospho-p38 MAPK in human hepatoma HepG2 cells. Environ. Toxicol. Pharm. 2012, 33, 181–190. [Google Scholar] [CrossRef]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug Resistance and the Solid Tumor Microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, X.D.; Liang, C.; Dong, L.; Qu, X.; Zhao, T. Simultaneous determination and pharmacokinetic study of gambogic acid and gambogenic acid in rat plasma after oral administration of Garcinia hanburyi extracts by LC-MS/MS. Biomed. Chromatogr. 2015, 29, 545–551. [Google Scholar] [CrossRef]
- Luo, Q.; Lin, T.Y.; Zhang, C.Y.; Zhu, T.; Wang, L.; Ji, Z.; Jia, B.; Ge, T.; Peng, D.; Chen, W. A novel glyceryl monoolein-bearing cubosomes for gambogenic acid: Preparation, cytotoxicity and intracellular uptake. Int. J. Pharm. 2015, 493, 30–39. [Google Scholar] [CrossRef]
- Lin, T.Y.; Fang, Q.; Peng, D.Y.; Huang, X.; Zhu, T.; Luo, Q.; Zhou, K.; Chen, W. PEGylated non-ionic surfactant vesicles as drug delivery systems for Gambogenic acid. Drug Deliv. 2013, 20, 277–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Chen, Y.J.; Peng, D.Y.; Li, Q.L.; Wang, X.S.; Wang, D.L.; Chen, W.D. Solid lipid nanoparticles as delivery systems for Gambogenic acid. Colloids Surf. B Biointerfaces 2013, 102, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.L.; Li, X.; Zhang, C.Y.; Pan, W.; Liang, Y.; Chen, Y.; Chen, W.; Liu, L.; Wang, X. Nanosuspensions as delivery system for gambogenic acid: Characterization and in vitro/in vivo evaluation. Drug Deliv 2015, 23, 2772–2779. [Google Scholar] [CrossRef]
- Webber, S.E. Polymer Micelles: An Example of Self-Assembling Polymers. J. Phys. Chem. B 1998, 102, 2618–2626. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, L.; Torchilin, V.P. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials 2013, 34, 1213–1222. [Google Scholar] [CrossRef]
- Tao, L.; Chan, J.W.; Uhrich, K.E. Drug loading and release kinetics in polymeric micelles: Comparing dynamic versus unimolecular sugar-based micelles for controlled release. J. Bioact. Compat. Polym. 2016, 31, 227–241. [Google Scholar] [CrossRef]
- Xu, J.; Qin, B.; Luan, S.; Qi, P.; Wang, Y.; Wang, K.; Song, S. Acid-labile poly(ethylene glycol) shell of hydrazone-containing biodegradable polymeric micelles facilitating anticancer drug delivery. J. Bioact. Compat. Polym. 2018, 33, 119–133. [Google Scholar] [CrossRef]
- Zhang, P.; Qian, X.; Zhang, Z.; Li, C.; Xie, C.; Wu, W.; Jiang, X. Supramolecular amphiphilic polymer-based micelles with seven-armed polyoxazoline coating for drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 5768–5777. [Google Scholar] [CrossRef]
- Ge, Z.S.; Liu, S.Y. Functional Block Copolymer Assemblies Responsive to Tumor and Intracellular Microenvironments for Site-specific Drug Delivery and Enhanced Imaging Performance. Chem. Soc. Rev. 2013, 42, 7289–7325. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, Y.; Wei, Y.; Cheng, G.; Ma, G. Thermo-triggered drug delivery from polymeric micelles of poly(N-isopropylacrylamide-co-acrylamide)-b-poly(n-butyl methacrylate) for tumor targeting. J. Bioact. Compat. Polym. 2014, 29, 301–317. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, Q.; Gao, X.; Chen, L.; Cao, Y.; Yu, S.; He, C.; Chen, X. pH-responsive poly(ethylene glycol)/Poly(L-lactide) supramolecular micelles based on host-guest interaction. ACS Appl. Mater. Interfaces 2015, 7, 8404–8411. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Chen, Y.; Wang, Y.; Li, H.; Han, H.; Chen, T.; Jin, Q.; Ji, J. pH- and NIR light-responsive polymeric prodrug micelles for hyperthermia-assisted site-specific chemotherapy to reverse drug resistance in cancer treatment. Small 2016, 12, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Xu, Y.; Li, G.; Hu, J.; Ma, B.; Yu, T.; Su, X.; Wang, Y. Redox and pH dual-responsive polymeric micelles with aggregation-induced emission feature for cellular imaging and chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 18489–18498. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, F. pH-Responsive Drug-Delivery Systems. Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Bae, J.W.; Choi, J.H.; Lee, J.S.; Park, K.D. Bioreducible cross-linked Pluronic micelles: pH-triggered release of doxorubicin and folate-mediated cellular uptake. J. Bioact. Compat. Polym. 2013, 28, 341–354. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, S.; Fan, P.; Wang, L.; Di, Y.; Lin, K.; Xiao, F.S. pH-responsive carrier system based on carboxylic acid modified mesoporous silica and polyelectrolyte for drug delivery. Chem. Mater. 2005, 17, 5999–6003. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, H.; Ge, W.; Wu, C.; Chen, D.; Li, Q.; Chen, Y.; Wang, X. Green and facile synthesis of highly biocompatible carbon nanospheres and their pH-responsive delivery of doxorubicin to cancer cells. RSC Adv. 2015, 5, 17532–17540. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M.; Bennett, C. PEG-co-polyvinyl pyridine coated magnetic mesoporous silica nanoparticles for Ph-responsive controlled release of doxorubicin. Int. J. Polym. Mater. 2015, 64, 570–577. [Google Scholar] [CrossRef]
- Oh, N.M.; Oh, K.T.; Youn, Y.S.; Lee, D.K.; Cha, K.H.; Lee, D.H.; Lee, E.S. Poly(L-aspartic acid) nanogels for lysosome-selective antitumor drug delivery. Colloids Surf. B Biointerfaces 2013, 101, 298–306. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.D.; Rao, Y.F.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef]
- Chen, S.; Bian, Q.; Wang, P.J.; Zheng, X.; Lv, L.; Dang, Z.; Wang, G. Photo, pH and redox multi-responsive nanogels for drug delivery and fluorescence cell imaging. Polym. Chem. 2017, 8, 6150–6157. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, J.; Zhao, X.B.; Tian, K.; Liu, P. Independent temperature and pH dual-responsive PMAA/PNIPAM microgels as drug delivery system: Effect of swelling behavior of the core and shell materials in fabrication process. Colloids Surf. A Physicochem. Eng. Aspects 2017, 526, 48–55. [Google Scholar] [CrossRef]
- Hiljanen-Vaninio, M.; Karjalainen, T.; Seppala, J. Biodegradable lactone copolymers. I. Characterization and mechanical behavior of ε–caprolactone and lactide copolymer. J. Appl. Polym. Sci. 1999, 59, 1281–1288. [Google Scholar] [CrossRef]
- Pan, W.D.; Liu, H.H.; Zhang, H.C.; Zhao, Y. Synthesis and properties of an acid-labile dual-sensitive ABCD star quaterpolymer. Polym. Chem. 2016, 7, 2870–2881. [Google Scholar] [CrossRef]
- Liu, H.H.; Li, C.X.; Tang, D.D.; An, X.; Guo, Y.; Zhao, Y. Multi-responsive graft copolymer micelles comprising acetal and disulfide linkages for stimuli-triggered drug delivery. J. Mater. Chem. B 2015, 3, 3959–3971. [Google Scholar] [CrossRef]
- Li, Z.L.; Huang, Y.S.; Xiong, X.Y.; Qin, X.; Luo, Y.Y. Synthesis, characterisation and in vitro release of paclitaxel-loaded polymeric micelles. Micro Nano Lett. 2017, 12, 191–194. [Google Scholar] [CrossRef]
- Xiao, L.; Xiong, X.Q.; Sun, X.H.; Zhu, Y.; Yang, H.; Chen, H.; Gan, L.; Xu, H.; Yang, X. Role of cellular uptake in the reversal of multidrug resistance by PEG-b-PLA polymeric micelles. Biomaterials 2011, 32, 5148–5157. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, X.Q.; Wan, J.L.; Xiao, L.; Gan, L.; Feng, Y.; Xu, H.; Yang, X. Cellular uptake and intracellular trafficking of PEG-b-PLA polymeric micelles. Biomaterials 2012, 33, 7233–7240. [Google Scholar] [CrossRef]
- Abdelbary, G.; Makhlouf, A. Adoption of polymeric micelles to enhance the oral bioavailability of dexibuprofen: Formulation, in-vitro evaluation and in-vivo pharmacokinetic study in healthy human volunteers. Pharm. Dev. Technol. 2014, 19, 717–727. [Google Scholar] [CrossRef]
- Yeh, J.C.; Hsu, Y.T.; Su, C.M.; Wang, M.C.; Lee, T.H.; Lou, S.L. Preparation and characterization of biocompatible and thermoresponsive micelles based on poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) grafted on polysuccinimide for drug delivery. J. Biomater. Appl. 2014, 29, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Altomare, L.; Bonetti, L.; Campiglio, C.E.; De Nardo, L.; Draghi, L.; Tana, F.; Fare, S. Biopolymer-based strategies in the design of smart medical devices and artificial organs. Int. J. Artif. Organs 2018, 41, 337–359. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Jin, Z.; Han, J.; Cho, S.; Nguyen, V.D.; Ko, S.Y.; Park, J.O.; Park, S. Preparation of HIFU-triggered tumor-targeted hyaluronic acid micelles for controlled drug release and enhanced cellular uptake. Colloids Surf. B Biointerfaces 2016, 143, 27–36. [Google Scholar] [CrossRef]
- Jesús, O.L.P.D.; Ihre, H.R.; Gagne, L.; Fréchet, J.M.; Szoka, F.C., Jr. Polyester dendritic systems for drug delivery applications: In vitro and in vivo evaluation. Bioconjugate Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef]









| Run | Polymer | I | M | Dp0 | C b | Mn,th c | Mn,gpc d | Pdi d | Mn,nmr (dppm) e |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PtBA | PCBP | tBA | 100 | 0.641 | 8920 | 9880 | 1.12 | 11,100 (81) |
| 2 | PtBA-b-PCL | PtBA | CL | 50 | 0.480 | 10,720 | 13,700 | 1.20 | 26,100 (132) |
| Formulation | GNA Solution | GNA-Loaded PMs |
|---|---|---|
| IC50 (μg/mL) | 7.029 | 5.040 |
| Pharmacokinetic Parameters | Formulations | |
|---|---|---|
| GNA Solution | GNA-Loaded PMs | |
| Cmax (mg L−1) | 5.77 ± 2.26 | 15.89 ± 6.86 * |
| AUC(0-t) (mg L−1∙min−1) | 97.64 ± 20.50 | 254.85 ± 67.31 * |
| AUC(0-∞) (mg L−1∙min−1) | 116.05 ± 21.59 | 287.53 ± 98.80 * |
| MRT(0-t) (min) | 45.49 ± 8.30 | 33.49 ± 12.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Chen, H.; Cao, F.; Peng, D.; Chen, W.; Zhang, C. Amphiphilic Block Copolymer Poly (Acrylic Acid)-B-Polycaprolactone as a Novel pH-sensitive Nanocarrier for Anti-Cancer Drugs Delivery: In-vitro and In-vivo Evaluation. Polymers 2019, 11, 820. https://doi.org/10.3390/polym11050820
Liu H, Chen H, Cao F, Peng D, Chen W, Zhang C. Amphiphilic Block Copolymer Poly (Acrylic Acid)-B-Polycaprolactone as a Novel pH-sensitive Nanocarrier for Anti-Cancer Drugs Delivery: In-vitro and In-vivo Evaluation. Polymers. 2019; 11(5):820. https://doi.org/10.3390/polym11050820
Chicago/Turabian StyleLiu, Huanhuan, Hong Chen, Fuhu Cao, Daiyin Peng, Weidong Chen, and Chuanling Zhang. 2019. "Amphiphilic Block Copolymer Poly (Acrylic Acid)-B-Polycaprolactone as a Novel pH-sensitive Nanocarrier for Anti-Cancer Drugs Delivery: In-vitro and In-vivo Evaluation" Polymers 11, no. 5: 820. https://doi.org/10.3390/polym11050820
APA StyleLiu, H., Chen, H., Cao, F., Peng, D., Chen, W., & Zhang, C. (2019). Amphiphilic Block Copolymer Poly (Acrylic Acid)-B-Polycaprolactone as a Novel pH-sensitive Nanocarrier for Anti-Cancer Drugs Delivery: In-vitro and In-vivo Evaluation. Polymers, 11(5), 820. https://doi.org/10.3390/polym11050820