Dextran-Coated Zinc-Doped Hydroxyapatite for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Materials
2.1.2. Dextran-Coated Zinc-Doped Hydroxyapatite
2.2. Characterizations
2.2.1. Ultrasonic Measurements
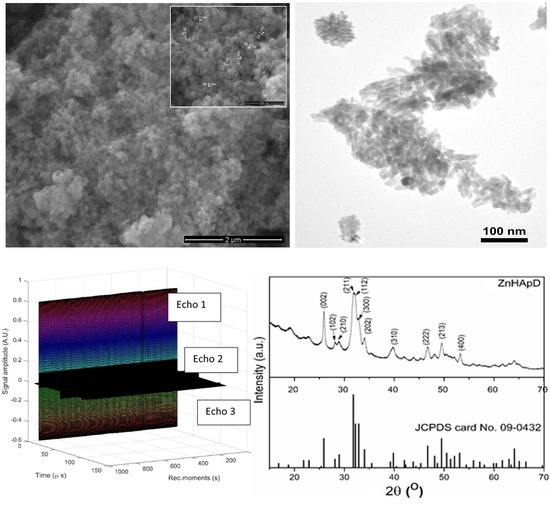
2.2.2. Structural and Morphological Characterizations.
2.3. Biological Studies
2.3.1. Cell Culture
2.3.2. Analysis of the Actin Cytoskeleton
2.4. Antibacterial Assays
2.5. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ranucci, E.; Ferruti, P.; Ranucci, E.; Ferruti, P. New basic multifunctional polymers: 5. Poly(esterthioetheramine)s by polyaddition of 2,2′-alkylenediimino diethanethiols to bisacrylic and bismethacrylic esters. Polymer 1991, 32, 2876–2879. [Google Scholar] [CrossRef]
- Prodan, A.M.; Beuran, M.; Turculet, C.S.; Popa, M.; Andronescu, E.; Bleotu, C.; Raita, S.M.; Soare, M.; Lupescu, O. In vitro evaluation of glycerol coated iron oxide nanoparticles in solution. Rom. Biotechnol. Lett. 2018, 23, 13901–13908. [Google Scholar]
- Rutherford, S.T.; Bassler, B.L. Bacterial Quorum Sensing: It’s Role in Virulence. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Chifiriuc, M.C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and Antimicrobial Activity of Silver-Doped Hydroxyapatite Nanoparticles. BioMed Res. Int. 2013, 2013, 916218. [Google Scholar] [CrossRef] [PubMed]
- Turculet, C.S.; Prodan, A.M.; Negoi, I.; Teleanu, G.; Popa, M.; Andronescu, E.; Beuran, M.; Stanciu, G.A.; Hristu, R.; Badea, M.L.; et al. Preliminary evaluation of the antifungal activity of samarium doped hydroxyapatite thin films. Rom. Biotechnol. Lett. 2018, 23, 13928–13932. [Google Scholar]
- Costescu, A.; Ciobanu, C.S.; Iconaru, S.L.; Ghita, R.V.; Chifiriuc, C.M.; Marutescu, L.G.; Predoi, D. Fabrication, Characterization, and Antimicrobial Activity, Evaluation of Low Silver Concentrations in Silver-Doped Hydroxyapatite Nanoparticles. J. Nanomater. 2013, 2013, 5. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, Structural and Biological Evaluation of Hydroxyapatite Doped with Zinc at Low Concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Bioceramic Layers with Antifungal Properties. Coatings 2018, 8, 276. [Google Scholar] [CrossRef]
- Pleszczyńska, M.; Wiater, A.; Bachanek, T.; Szczodrak, J. Enzymes in therapy of biofilm-related oral diseases. Biotechnol. Appl. Biochem. 2017, 64, 337–346. [Google Scholar] [CrossRef]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 3, 661–669. [Google Scholar]
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontology 2000, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Beikler, T.; Flemming, T.F. Oral biofilm-associated diseases: Trends and implications for quality of life, systemic health and expenditures. Periodontology 2000, 55, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.L.; Hasson, H. Fluoride supplements, dental caries and fluorosis: A systematic review. J. Am. Dent. Assoc. 2008, 139, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Loë, H. Oral hygiene in the prevention of caries and periodontal disease. Int. Dent. J. 2000, 50, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.G.; Rosalen, P.L.; Falsetta, M.L.; Koo, H. Natural Products in Caries Research: Current (Limited) Knowledge, Challenges and Future Perspective. Caries Res. 2011, 45, 243–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brading, M.G.; Marsh, P.D. The oral environment: The challenge for antimicrobials in oral care products. Int. Dent. J. 2003, 53, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Muller, N.; Cionca, N. The epidemiology of periimplantitis. Clin. Oral Implant. Res. 2012, 23, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Grischke, J.; Eberhard, J.; Stiesch, M. Antimicrobial Dental Implant Functionalization Strategies—A Systematic Review. Dent. Mater. J. 2016, 35, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Allaker, R.P.; Douglas, C.W.I. Novel anti-microbial therapies for dental plaque-related diseases. Int. J. Antimicrob. Agents 2009, 33, 8–13. [Google Scholar] [CrossRef]
- Antipa, C.; Popa, M.; Măruțescu, M.; Bleotu, C.; Lazar, V.; Bertesteanu, S.; Grigore, R.; Bezirtzoglou, E.; Ruța, S.M. Virulence Profiles of Bacterial Strains Isolated from Periodontal Lesions. Rom. Biotechnol. Lett. 2015, 20, 10662–10669. [Google Scholar]
- Thallinger, B.; Prasetyo, E.N.; Nyanhango, G.S.; Guebitz, G.M. Antimicrobial enzymes: An emerging strategy to fight microbes and microbial biofilms. Biotechnol. J. 2013, 8, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Asaga, E.; Goto, N. Roles of Streptococcus mutans dextranase anchored to the cell wall by sortase. Oral Microbiol. Immunol. 2004, 19, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Morisaki, H.; Goto, N. Molecular characterization of dextranase from Streptococcus rattus. Microbiol. Immunol. 2004, 48, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Critchley, P. The Extracellular Polysaccharide Produced from Sucrose by A Cariogenic Streptococcus. Arch. Oral Biol. 1966, 11, 1039–1042. [Google Scholar] [CrossRef]
- Carlsson, J.; Egelberg, J. Effect of Diet on Early Plaque Formation in Man. Odontol. Revy 1965, 16, 112–125. [Google Scholar] [PubMed]
- Gibbons, R.J.; Banghart, S. Synthesis of Dextran by Cariogenic Bacteria and Its Presence in Human Dental Plaque. Arch. Oral Biol. 1967, 12, 11–24. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Fitzgeraldr, J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in formation of microbial dental plaque. J. Bact. 1969, 98, 341–396. [Google Scholar] [PubMed]
- Rblla, G. Adsorption of Dextran to Saliva-Treated Hydroxyapatite. Arch. Oral Biol. 1971, 16, 527–533. [Google Scholar]
- Colby, S.M.; Whiting, G.C.; Tao, L.; Russell, R.R. Insertional inactivation of the Streptococcus mutans dexA (dextranase) gene results in altered adherence and dextran catabolism. Microbiology 1995, 141, 2929–2936. [Google Scholar] [CrossRef] [Green Version]
- Khalikova, E.; Susi, P.; Korpela, T. Microbial Dextran-Hydrolyzing Enzymes: Fundamentals and Applications. Microbiol. Mol. Biol. Rev. 2005, 69, 306–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, G.J. Some properties of a dextranglucosidase isolated from oral streptococci and its use in studies on dextran synthesis. J. Dent. Res. 1972, 51, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Schachtele, C.F.; Staat, R.H.; Harlander, S.K. Dextranases from oral bacteria: Inhibition of water-insoluble glucan production and adherence to smooth surfaces by Streptococcus mutans. Infect. Immun. 1975, 12, 309–317. [Google Scholar] [PubMed]
- Staat, R.H.; Schachtele, C.F. Analysis of the dextranase activity produced by an oral strain of Bacteroides ochraceus. J. Dent. Res. 1976, 55, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, B.; König, K.G.; Mühlemann, H.R.; Regolati, B. Caries and plaque inhibition by mutanase in rats. Arch. Oral Biol. 1969, 14, 555–558. [Google Scholar] [CrossRef]
- Caldwell, R.C.; Sandham, H.J.; Mann, W.V., Jr.; Finn, S.B.; Formicola, A.J. The Effect of a Dextranase Mouthwash on Dental Plaque in Young Adults and Children. J. Am. Dent. Assoc. 1971, 82, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lobene, R.R. A Clinical Stuoy of the Effect of Dextranase on Human Dental Plaque. J. Am. Dent. Assoc. 1971, 82, 132–135. [Google Scholar] [CrossRef]
- Stephen, K.W.; Saxton, C.A.; Jones, C.L.; Ritchie, J.A.; Morrison, T. Control of Gingivitis and Calculus by a Dentifrice Containing a Zinc Salt and Triclosan. J. Periodontol. 1990, 61, 674–679. [Google Scholar] [CrossRef]
- Harrap, G.J.; Saxton, C.A.; Best, J.S. Inhibition of plaque growth by zinc salts. J. Periodont. Res. 1984, 18, 634–642. [Google Scholar] [CrossRef]
- Saxton, C.A.; Harrap, G.J.; Lloyd, A.M. The effect of dentifrices containing zinc citrate on plaque growth and oral zinc levels. J. Clin. Periodontol. 1986, 13, 301–306. [Google Scholar] [CrossRef]
- Kornman, K.S. The role of supragingival plaque in the prevention and treatment of periodontol disease. J. Periodont. Res. 1986, 21, 307–313. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.L.; Deniaud, A.; Michaud-Soret, I.; Guégan, R.; Motelica-Heino, M.; Predoi, D. Structural and Biological Assessment of Zinc Doped Hydroxyapatite Nanoparticles. J. Nanomater. 2016, 2016, 1062878. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Constantin, L.V.; Predoi, D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res. Lett. 2012, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, G.; Bargan, A.M.; Luca, C. New cerium (IV)-substituted hydroxyapatite nanoparticles: Preparation and characterization. Ceram. Int. 2015, 41, 12192–12201. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional polymers base on dextran. Adv. Polym. Sci. 2006, 205, 199–291. [Google Scholar]
- Robyt, J.F. Encyclopedia of Polymer Science and Technology, 2nd ed.; Kroschwitz, J.I., Ed.; John Wiley & Sons: New York, NY, USA, 1987; Volume 3, pp. 752–767. [Google Scholar]
- Allene, J.; Mark, H.F. Encyclopedia in Polymer Science and Engineering Technology; Mark, H.F., Gaylord, N.G., Bikales, N.M., Eds.; John Wiley & Sons: New York, NY, USA, 1966; pp. 805–824. [Google Scholar]
- Suflet, D.M.; Chitanu, G.C.; Desbrières, J. Phosphorylated polysaccharides. 2. Synthesis and properties of phosphorylated Dextran. Carbohydr. Polym. 2010, 82, 1271–1277. [Google Scholar] [CrossRef]
- Romero, H.A.M.; Ruacho, J.M.; Pérez, C.A.M.; Casillas, P.E.G. Synthesis of Hydroxyapatite Nanoparticles in Presence of a Linear Polysaccharide. J. Mater. 2013, 2013, 683268. [Google Scholar]
- Stricker, J.; Falzone, T.; Gardel, M.L. Mechanics of the F-actin cytoskeleton. J. Biomech. 2010, 43, 9–14. [Google Scholar] [CrossRef]
- Prakash, H.; Sidhu, S.S.; Sundaram, K.R. Prevalence of Dental Caries among delhi school chidren. J. Ind. Dent. Assoc. 1999, 70, 12–14. [Google Scholar]
- Abdullah, S.; Qazi, H.S.; Maxood, A. Dental caries status in 6–9 years old children. Pak. Oral Dent. J. 2008, 28, 107–112. [Google Scholar]
- Raju, K.S.; Anitha, L. Isolation and Identification of Oral flora from individuals belonging to ages 7 to 16 years. Res. J. Sci. IT Manag. RJSITM 2015, 4, 1–7. [Google Scholar]
- De Kraker, M.E.; Davey, P.G.; Grundmann, H. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: Estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011, 8, e1001104. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D. Extraintestinal pathogenic Escherichia coli: An update on antimicrobial resistance, laboratory diagnosis and treatment. Expert Rev. Anti-Infect. Ther. 2012, 10, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Porphyromonas gingivalis-host interactions: Open war or intelligent guerilla tactics? Microbes Infect. 2009, 11, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Van Winkelhoff, A.J.; Herrera Gonzales, D.; Winkel, E.G.; Dellemijn-Kippuw, N.; Vandenbroucke-Grauls, C.M.; Sanz, M. Antimicrobial resistance in the subgingival microflora in patients with adult periodontitis. A comparison between The Netherlands and Spain. J. Clin. Periodontol. 2000, 27, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sanai, Y.; Persson, G.R.; Starr, J.R.; Luis, H.S.; Bernardo, M.; Leitao, J.; Roberts, M.C. Presence and antibiotic resistance of Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens in children. J. Clin. Periodontol. 2002, 29, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Robertson, D.M.; Tang, K.; Jackson, M.S.; MacKenzie, D.; Bagg, J. Staphylococcus aureus in the oral cavity: At hree-year retrospective analysis of clinical laboratory data. Br. Dent. J. 2003, 195, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Berman, K.S.; Knoettner, P.; Kapsimalis, B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch. Oral Biol. 1966, 11, 549–560. [Google Scholar] [CrossRef]














© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, D.; Iconaru, S.L.; Predoi, M.V. Dextran-Coated Zinc-Doped Hydroxyapatite for Biomedical Applications. Polymers 2019, 11, 886. https://doi.org/10.3390/polym11050886
Predoi D, Iconaru SL, Predoi MV. Dextran-Coated Zinc-Doped Hydroxyapatite for Biomedical Applications. Polymers. 2019; 11(5):886. https://doi.org/10.3390/polym11050886
Chicago/Turabian StylePredoi, Daniela, Simona Liliana Iconaru, and Mihai Valentin Predoi. 2019. "Dextran-Coated Zinc-Doped Hydroxyapatite for Biomedical Applications" Polymers 11, no. 5: 886. https://doi.org/10.3390/polym11050886
APA StylePredoi, D., Iconaru, S. L., & Predoi, M. V. (2019). Dextran-Coated Zinc-Doped Hydroxyapatite for Biomedical Applications. Polymers, 11(5), 886. https://doi.org/10.3390/polym11050886