3D Bioprinted Nanocellulose-Based Hydrogels for Tissue Engineering Applications: A Brief Review
Abstract
:1. Introduction
2. Nanocellulose: Synthesis, Mechanical Properties, Biodegradation and Biocompatibility
3. 3D Bioprinting Approach for Hydrogel Fabrication
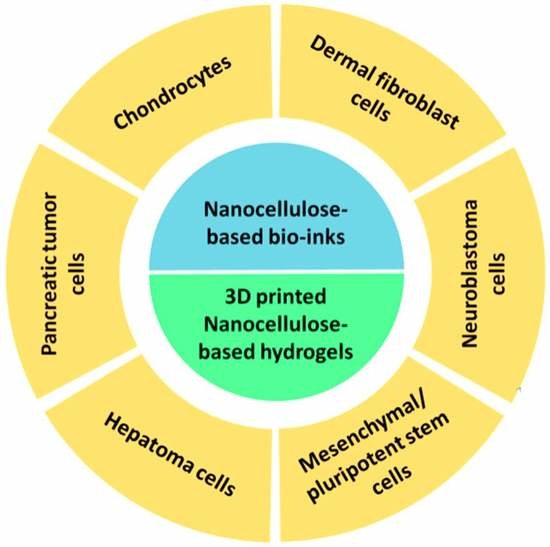
4. 3D Bioprinted Nanocellulose-Based Hydrogels: Properties and Biomedical Applications
5. Conclusions and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| CNC | cellulose nanocrystal |
| CNF | cellulose nanofiber |
| BNC | bacterial nanocellulose |
| TEMPO | (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl |
| ECM | extra-cellular matrix |
| hNCs | human nasal chondrocytes |
| rACs | rabbit auricular chondrocytes |
| hBMSCs | human bone marrow–derived mesenchymal stem cells |
| HA | hyaluronic acid |
| iPSCs | human pluripotent stem cells |
| PU | polyurethane |
| GM | gelatin methacrylate |
| GGM | galactoglucomannan methacrylate |
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa, A., Jr.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.P.; Selvakumar, R.; Suresh kumar, P.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef] [Green Version]
- Isogai, A.; Saito, T.; Fukuzumi, H. Tempo-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.; Dufresne, A.; Thomas, S.; Gałęski, A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- DeFrance, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Aubert, J.-P.; Béguin, P. The biological degradation of cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar]
- Yeoman, C.J.; Han, Y.; Dodd, D.; Schroeder, C.M.; Mackie, R.I.; Cann, I.K.O. Chapter 1—Thermostable enzymes as biocatalysts in the biofuel industry. In Advances in Applied Microbiology; Academic Press: New York, NY, USA, 2010; Volume 70, pp. 1–55. [Google Scholar]
- Czaja, W.; Kyryliouk, D.; DePaula, C.A.; Buechter, D.D. Oxidation of γ-irradiated microbial cellulose results in bioresorbable, highly conformable biomaterial. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Singh, G.; Chandoha-Lee, C.; Zhang, W.; Renneckar, S.; Vikesland, P.J.; Pruden, A. Biodegradation of nanocrystalline cellulose by two environmentally-relevant consortia. Water Res. 2016, 104, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Ramadurai, K.W. 3-dimensional device fabrication: A bio-based materials approach. In 3D Printing and Bio-Based Materials in Global Health: An Interventional Approach to the Global Burden of Surgical Disease in Low- and Middle-Income Countries; Bhatia, S.K., Ramadurai, K.W., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 39–61. [Google Scholar]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A review of 3D printing technology for medical applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Donderwinkel, I.; van Hest, J.C.M.; Cameron, N.R. Bio-inks for 3D bioprinting: Recent advances and future prospects. Polym. Chem. 2017, 8, 4451–4471. [Google Scholar] [CrossRef]
- Maiti, B.; Díaz Díaz, D. 3D printed polymeric hydrogels for nerve regeneration. Polymers 2018, 10, 1041. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Siqueira, G.; Zimmermann, T.; Mathew, A.P. 3D printing of nano-cellulosic biomaterials for medical applications. Curr. Opin. Biomed. Eng. 2017, 2, 29–34. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Sandler, N.; Willför, S.; Xu, C. Three-dimensional printing of wood-derived biopolymers: A review focused on biomedical applications. ACS Sustain. Chem. Eng. 2018, 6, 5663–5680. [Google Scholar] [CrossRef]
- Xu, C.; Molino, B.Z.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S.; et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B 2018, 6, 7066–7075. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Karabulut, E.; Pernevik, E.; Enoksson, P.; Gatenholm, P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 2018, 189, 22–30. [Google Scholar] [CrossRef]
- Fall, A.B.; Lindström, S.B.; Sundman, O.; Ödberg, L.; Wågberg, L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir 2011, 27, 11332–11338. [Google Scholar] [CrossRef]
- Siqueira, G.; Kokkinis, D.; Libanori, R.; Hausmann, M.K.; Gladman, A.S.; Neels, A.; Tingaut, P.; Zimmermann, T.; Lewis, J.A.; Studart, A.R. Cellulose nanocrystal inks for 3D printing of textured cellular architectures. Adv. Funct. Mater. 2017, 27, 1604619. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef]
- Nascimento, D.M.; Nunes, Y.L.; Figueirêdo, M.C.B.; DeAzeredo, H.M.C.; Aouada, F.A.; Feitosa, J.P.A.; Rosa, M.F.; Dufresne, A. Nanocellulose nanocomposite hydrogels: Technological and environmental issues. Green Chem. 2018, 20, 2428–2448. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Ávila, H.M.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1–2, 22–35. [Google Scholar] [CrossRef]
- Möller, T.; Amoroso, M.; Hägg, D.; Brantsing, C.; Rotter, N.; Apelgren, P.; Lindahl, A.; Kölby, L.; Gatenholm, P. In vivo chondrogenesis in 3D bioprinted human cell-laden hydrogel constructs. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1227. [Google Scholar] [CrossRef]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q.; Becher, J.; Möller, S.; Schnabelrauch, M.; Zenobi-Wong, M. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng. Part A 2013, 20, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Henriksson, I.; Gatenholm, P.; Hägg, D.A. Increased lipid accumulation and adipogenic gene expression of adipocytes in 3D bioprinted nanocellulose scaffolds. Biofabrication 2017, 9, 015022. [Google Scholar] [CrossRef]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage tissue engineering by the 3D bioprinting of ips cells in a nanocellulose/alginate bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef]
- Chen, R.-D.; Huang, C.-F.; Hsu, S.-H. Composites of waterborne polyurethane and cellulose nanofibers for 3D printing and bioapplications. Carbohydr. Polym. 2019, 212, 75–88. [Google Scholar] [CrossRef]
- Xu, W.; Molino, B.Z.; Cheng, F.; Molino, P.J.; Yue, Z.; Su, D.; Wang, X.; Willför, S.; Xu, C.; Wallace, G.G. On low-concentration inks formulated by nanocellulose assisted with gelatin methacrylate (gelma) for 3D printing toward wound healing application. ACS Appl. Mater. Interfaces 2019, 11, 8838–8848. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Yang, P.; Långvik, O.; Wang, X.; Zhang, Y.; Cheng, F.; Österberg, M.; Willför, S.M.; Xu, C. Surface engineered biomimetic inks based on uv cross-linkable wood biopolymers for 3D printing. ACS Appl. Mater. Interfaces 2019, 11, 12389–12400. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, J.; Jiang, Y.; Zhang, Q.; Shi, H.; Liu, D. 3D printing process of oxidized nanocellulose and gelatin scaffold. J. Biomater. Sci. Polym. Ed. 2018, 29, 1498–1513. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.Y.; Wenger, A.C.; Tam, K.C.; Tang, X. 3D bioprinting of liver-mimetic construct with alginate/cellulose nanocrystal hybrid bioink. Bioprinting 2018, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Kyle, S.; Thomas, B.; Badiei, N.; Hawkins, K.; Whitaker, I.S. Printability of pulp derived crystal, fibril and blend nanocellulose-alginate bioinks for extrusion 3D bioprinting. Biofabrication 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]





| Hydrogel Composition | Bioink | 3D Printing Feed Rate; Nozzle Size; and Pressure | Crosslinking Condition | Mechanical/Electrical Properties | Mammalian Cell Biocompatibility | Biomedical Application | Ref. |
|---|---|---|---|---|---|---|---|
| CNF | No | 8 mm/s; 0.20 mm; 50 kPa | 0.01% 1,4-butanediol diglycidyl ether; 50 °C; 2 h | Compressive Young’s moduli: 3.45–7.44 kPa | Human fibroblast cells | Wound healing | [26] |
| CNF/alginate (90/10, 80/20, 70/30, 60/40) | Yes | 5–20 mm/s; 0.30 mm; 20–60 kPa | 90 mM CaCl2; 10 min | Compressive stress: 22–33 kPa at 30% strain | Human chondrocyte cells; viability—73% (Day 1), 86% (Day 7) | Cartilage tissue engineering | [33] |
| CNF/alginate (2/0.5) | Yes | 5 mm/s; 0.15 mm; 4 kPa | 100 mM CaCl2; 10 min | - | Human and rabbit chondrocyte cells; viability—96% (human), 99% (rabbit) | Cartilage tissue engineering | [34] |
| CNF/alginate (CELLINK Bioink, Sweden) | Yes | - | 100 mM CaCl2; 5 min | Compressive stress: 15–39 kPa at 40% strain | Human chondrocyte and mesenchymal stem cells | Tissue engineering | [35] |
| CNF/alginate (1.36/1); CNF/alginate sulfate (1.36/0.5) | Yes | 0.16–0.41 mm; 6–74 kPa | 100 mM CaCl2; 12 min | Shear storage modulus: 14.6 kPa | Bovine chondrocyte cells; viability > 85% | Cartilage tissue engineering | [37] |
| CNF/alginate (80/20); CNF/hyaluronan (80/20, 70/30) | Yes | 17–20 kPa | CNF/alginate—100 mM CaCl2; 10 min; CNF/hyaluronan—0.001% H2O2; 5 min | Compression stress: 19–55 kPa at 40% strain | Mouse mesenchymal stem cells; viability—95% (Day 7) | Tissue engineering | [39] |
| CNF/alginate (60/40); CNF/hyaluronan (80/20) | Yes | 10–20 mm/s; 0.30 mm; 20–30 kPa | CNF/alginate—100 mM CaCl2; 5 min; CNF/hyaluronan—0.001% H2O2; 5 min | - | Human pluripotent stem cells | Cartilage tissue engineering | [40] |
| CNF/carbon nanotube (80/20) | No | 10 mm/s; 0.30 mm; 65 kPa | - | Conductivity: 3.8 × 10−1 S/cm | Human neuroblastoma cells; viability > 95% | Neural tissue engineering | [27] |
| CNF/polyurethane (9/29) | No | 7–10 mm/s; 0.16 mm and 0.21 mm; 50–200 kPa | - | Compression storage modulus: 1.57 MPa | Mouse and human fibroblast cells | Tissue engineering | [41] |
| CNF/gelatin methacrylate (5/1, 2/1, 9/10) | No | 16–33 mm/s; 0.16 mm and 0.21 mm; 65–80 kPa | 0.5% Irgacure 2959; 10 mW/cm2 UV (320–390 nm); 5 min | Compressive Young’s moduli: 2.5–5 kPa | Mouse fibroblast cells; viability > 90% | Wound healing | [42] |
| CNF/galactoglucomannan methacrylate (1/1, 1/2, 1/3) | No | 5 mm/s; 0.21 mm | 0.5% Irgacure 2959; 10 mW/cm2 UV (320–390 nm); 5 min | Compressive Young’s moduli: 2.5–22.5 kPa | Human dermal fibroblast and pancreatic tumor cells; viability > 80% (fibroblast), > 60% (pancreatic) | Tissue engineering | [43] |
| CNC/gelatin | No | 5–15 mm/s; 0.21 mm and 0.41 mm | 4 °C and 20 °C; 0.25–24 h | Compressive yield deformation at 20% strain | Mouse fibroblast cells | Tissue engineering | [44] |
| CNC/alginate (1/1, 1/2, 2/1, 3/2) | Yes | 25 mm/s; 0.11 mm; 34–172 kPa | 1% CaCl2; 10 min | Shear storage moduli: 8–300 Pa | Mouse fibroblast and human hepatoma cells; viability—71% (fibroblast), 67% (hepatoma) | Tissue engineering | [45] |
| CNC/alginate (4/1); CNF/alginate (4/1); CNC-CNF/alginate (4/1); | Yes | 0.61 mm | 0.5–1 M CaCl2; 2–4 min | Compressive Young’s modulus: 52.6 kPa | Human chondrocyte cells; viability > 71% | Cartilage tissue engineering | [46] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athukoralalage, S.S.; Balu, R.; Dutta, N.K.; Roy Choudhury, N. 3D Bioprinted Nanocellulose-Based Hydrogels for Tissue Engineering Applications: A Brief Review. Polymers 2019, 11, 898. https://doi.org/10.3390/polym11050898
Athukoralalage SS, Balu R, Dutta NK, Roy Choudhury N. 3D Bioprinted Nanocellulose-Based Hydrogels for Tissue Engineering Applications: A Brief Review. Polymers. 2019; 11(5):898. https://doi.org/10.3390/polym11050898
Chicago/Turabian StyleAthukoralalage, Sandya S., Rajkamal Balu, Naba K. Dutta, and Namita Roy Choudhury. 2019. "3D Bioprinted Nanocellulose-Based Hydrogels for Tissue Engineering Applications: A Brief Review" Polymers 11, no. 5: 898. https://doi.org/10.3390/polym11050898
APA StyleAthukoralalage, S. S., Balu, R., Dutta, N. K., & Roy Choudhury, N. (2019). 3D Bioprinted Nanocellulose-Based Hydrogels for Tissue Engineering Applications: A Brief Review. Polymers, 11(5), 898. https://doi.org/10.3390/polym11050898