Cellulose Nanofibrils and Tubular Halloysite as Enhanced Strength Gelation Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Nanofibrils
2.3. Characterization of the Employed Materials
2.4. Preparation of Reinforced Silica Gels
2.5. Gel Strength Measurements
3. Results
3.1. Characterization of Cellulose Nanofibrils
3.2. Characterization of Halloysite Nanotubes
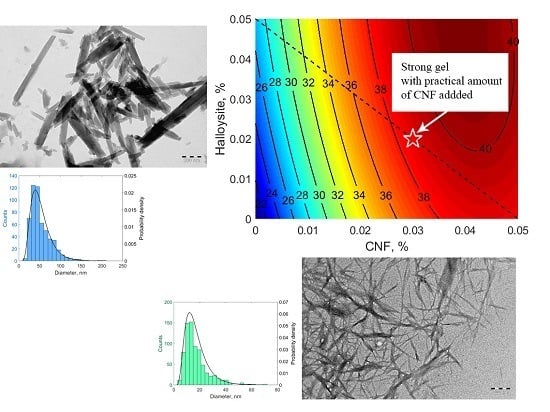
3.3. Gelation Kinetics and Gel Strength
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Experimental conditions | a1 | a2 | a3 |
|---|---|---|---|
| Figure 5 | |||
| No additives; gelation time 4 h | 412.89 1 | 5.7940 | 0.12076 |
| No additives; gelation time 7.5 h | 101.80 | 4.3339 | 0.17328 |
| 0.05% Praestol 2540; gelation time 4 h | 19395 | 8.6720 | 0.051108 |
| 0.05% Praestol 2540; gelation time 7.5 h | 44238 | 9.6360 | 0.046180 |
| Figure 6 | |||
| No additives | 24.940 | 6.0196 | 0.59552 |
| 0.025% CNF2 | 43.437 | 5.7342 | 0.58211 |
| 0.05% CNF | 44.446 | 7.6086 | 0.69905 |
| 0.025% Halloysite | 4322.1 | 7.2512 | 0.059066 |
| 0.025% CNF + 0.025% Halloysite | 44.246 | 7.2674 | 0.68397 |
| 0.025% CNF + 0.05% Halloysite | 43.803 | 8.6246 | 0.74702 |
| 0.05% CNF + 0.025% Halloysite | 45.458 | 7.4676 | 0.69053 |
| 0.05% CNF + 0.05% Halloysite | 48.352 | 6.0964 | 0.61393 |



References
- Almohsin, A.; Huang, J.; Karadkar, P.; Bataweel, M. Nanosilica Based Fluid System for Water Shut-Off. In 22nd World Petroleum Congress, 9–13 July 2017, Istanbul, Turkey; World Petroleum Congress: Houston, TX, USA, 2017. [Google Scholar]
- Huang, J.; Al-Mohsin, A.; Bataweel, M.; Karadkar, P.; Li, W.; Shaikh, A. Systematic Approach to Develop a Colloidal Silica Based Gel System for Water Shut-Off. In SPE Middle East Oil & Gas Show and Conference, 6–9 March 2017, Manama, Kingdom of Bahrain; Society of Petroleum Engineers: Richardson, TX, USA, 2017. [Google Scholar]
- Hunt, J.D.; Ezzedine, S.M.; Bourcier, W.; Roberts, S. Kinetics of the Gelation of Colloidal Silica at Geothermal Conditions, and Implications for Reservoir Modification and Management. In Stanford Geothermal Workshop, Palo Alto, California, USA, 11–13 February 2013; Lawrence Livermore National Lab (LLNL): Livermore, CA, USA, 2013. [Google Scholar]
- Zhu, D.; Wei, L.; Wang, B.; Feng, Y. Aqueous Hybrids of Silica Nanoparticles and Hydrophobically Associating Hydrolyzed Polyacrylamide Used for EOR in High-Temperature and High-Salinity Reservoirs. Energies 2014, 7, 3858–3871. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dai, C.; Wang, K.; Zou, C.; Gao, M.; Fang, Y.; Zhao, M.; Wu, Y.; You, Q. Study on a Novel Cross-Linked Polymer Gel Strengthened with Silica Nanoparticles. Energy Fuels 2017, 31, 9152–9161. [Google Scholar] [CrossRef]
- Asadizadeh, S.; Ayatollahi, S.; ZareNezhad, B. Performance Evaluation of a New Nanocomposite Polymer Gel for Water Shutoff in Petroleum Reservoirs. J. Dispers. Sci. Technol. 2018, 36, 1–9. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of Cellulose Nanofibrils: A Review of Recent Advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Qin, Y.; Qiu, X.; Zhu, J.Y. Understanding Longitudinal Wood Fiber Ultra-Structure for Producing Cellulose Nanofibrils Using Disk Milling with Diluted Acid Prehydrolysis. Sci. Rep. 2016, 6, 35602. [Google Scholar] [CrossRef] [PubMed]
- Boufi, S.; Chaker, A. Easy Production of Cellulose Nanofibrils from Corn Stalk by a Conventional High Speed Blender. Ind. Crop. Prod. 2016, 93, 39–47. [Google Scholar] [CrossRef]
- Xiang, Z.; Gao, W.; Chen, L.; Lan, W.; Zhu, J.Y.; Runge, T. A Comparison of Cellulose Nanofibrils Produced from Cladophora Glomerata Algae and Bleached Eucalyptus Pulp. Cellulose 2016, 23, 493–503. [Google Scholar] [CrossRef]
- Dufresne, A. Cellulose Nanomaterial Reinforced Polymer Nanocomposites. Curr. Opin. Colloid Interface Sci. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Miao, X.; Tian, F.; Lin, J.; Li, H.; Li, X.; Bian, F.; Zhang, X. Tuning the Mechanical Properties of Cellulose Nanofibrils Reinforced Polyvinyl Alcohol Composites: Via Altering the Cellulose Polymorphs. RSC Adv. 2016, 6, 83356–83365. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, W.; Dai, L.; Si, C.; Ni, Y. Fabrication of Thermo-And pH-Sensitive Cellulose Nanofibrils-Reinforced Hydrogel with Biomass Nanoparticles. Carbohydr. Polym. 2019, 215, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of Morphology and Size of Halloysite Nanotubes on Functional Pectin Bionanocomposites for Food Packaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A.; McPhee, D.J. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, M.; Zabihi, O.; Ahmadi, M.; Li, Q.; Depalmeanar, A.; Naebe, M. Nano-Enhanced Interface in Carbon Fibre Polymer Composite Using Halloysite Nanotubes. Compos. Part A Appl. Sci. Manuf. 2018, 109, 115–123. [Google Scholar] [CrossRef]
- Shuttleworth, P.S.; Díez-Pascual, A.M.; Marco, C.; Ellis, G. Flexible Bionanocomposites from Epoxidized Hemp Seed Oil Thermosetting Resin Reinforced with Halloysite Nanotubes. J. Phys. Chem. B 2017, 121, 2454–2467. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Halloysite Nanotubes as Support for Metal-Based Catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Glotov, A.; Levshakov, N.; Stavitskaya, A.; Artemova, M.; Gushchin, P.; Ivanov, E.; Vinokurov, V.; Lvov, Y. Templated Self-Assembly of Ordered Mesoporous Silica on Clay Nanotubes. Chem. Commun. 2019, 55, 5507–5510. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Gentile, P.; Ferreira, A.M.; Cometa, S.; De Giglio, E. Insight into Halloysite Nanotubes-Loaded Gellan Gum Hydrogels for Soft Tissue Engineering Applications. Carbohydr. Polym. 2017, 163, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Ferreira, C.; Veiga, F.; Ribeiro, A.J.; Panchal, A.; Lvov, Y.; Agarwal, A. Halloysite Clay Nanotubes for Life Sciences Applications: From Drug Encapsulation to Bioscaffold. Adv. Colloid Interface Sci. 2018, 257, 58–70. [Google Scholar] [CrossRef]
- Liu, M.; Fakhrullin, R.; Novikov, A.; Panchal, A.; Lvov, Y. Tubule Nanoclay-Organic Heterostructures for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800419. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Halloysite Nanotubes for Cleaning, Consolidation and Protection. Chem. Rec. 2018, 18, 940–949. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Leng, F.; Huang, L.; Wang, Z.; Tian, W. Recent Advances on Surface Modification of Halloysite Nanotubes for Multifunctional Applications. Appl. Sci. 2017, 7, 1215. [Google Scholar] [CrossRef]
- Shchukina, E.; Grigoriev, D.; Sviridova, T.; Shchukin, D. Comparative Study of the Effect of Halloysite Nanocontainers on Autonomic Corrosion Protection of Polyepoxy Coatings on Steel by Salt-Spray Tests. Prog. Org. Coat. 2017, 108, 84–89. [Google Scholar] [CrossRef]
- Shchukina, E.; Shchukin, D.; Grigoriev, D. Effect of Inhibitor-Loaded Halloysites and Mesoporous Silica Nanocontainers on Corrosion Protection of Powder Coatings. Prog. Org. Coat. 2017, 102, 60–65. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The Application of Halloysite Tubule Nanoclay in Drug Delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef]
- Liu, M.; Chang, Y.; Yang, J.; You, Y.; He, R.; Chen, T.; Zhou, C. Functionalized Halloysite Nanotube by Chitosan Grafting for Drug Delivery of Curcumin to Achieve Enhanced Anticancer Efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef]
- Yong, C.; Mei, C.; Guan, M.; Wu, Q.; Han, J.; Sun, X. A Comparative Study of Different Nanoclay-Reinforced Cellulose Nanofibril Biocomposites with Enhanced Thermal and Mechanical Properties. Compos. Interfaces 2018, 25, 301–315. [Google Scholar] [CrossRef]
- Novikov, A.A.; Anikushin, B.M.; Petrova, D.A.; Konstantinova, S.A.; Mel’nikov, V.B.; Vinokurov, V.A. Acid and Oxidative Treatment of Raw Material for the Production of Nanofibrillar Cellulose. Chem. Technol. Fuels Oils 2018, 54, 564–568. [Google Scholar] [CrossRef]
- TAPPI 211 om-93. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C; Committee of the Process and Product Quality Division: TAPPI, Atlanta, GA, USA, 1996. [Google Scholar]
- Abubakarova, A.S.; Khadisova, Z.T.; Aleksandrova, E.A.; Krasavtsev, B.E. Study of Structural-Mechanical Properties of Paraffin-Containing Petroleum Products. Chem. Technol. Fuels Oils 2014, 50, 149–155. [Google Scholar] [CrossRef]
- Ferrari, F.; Bertoni, M.; Caramella, C.; La Manna, A. Description and Validation of an Apparatus for Gel Strength Measurements. Int. J. Pharm. 1994, 109, 115–124. [Google Scholar] [CrossRef]
- Rodnova, V.Y. Gel-Forming Compositions Based on the Alkaline Silica Gel for the Water Shutoff. Ph.D. Thesis, Gubkin University, Moscow, Russia, 20 September 2018. [Google Scholar]
- Lee, C.S.; Robinson, J.; Chong, M.F. A Review on Application of Flocculants in Wastewater Treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent Advance in Research on Halloysite Nanotubes-Polymer Nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinokurov, V.; Novikov, A.; Rodnova, V.; Anikushin, B.; Kotelev, M.; Ivanov, E.; Lvov, Y. Cellulose Nanofibrils and Tubular Halloysite as Enhanced Strength Gelation Agents. Polymers 2019, 11, 919. https://doi.org/10.3390/polym11050919
Vinokurov V, Novikov A, Rodnova V, Anikushin B, Kotelev M, Ivanov E, Lvov Y. Cellulose Nanofibrils and Tubular Halloysite as Enhanced Strength Gelation Agents. Polymers. 2019; 11(5):919. https://doi.org/10.3390/polym11050919
Chicago/Turabian StyleVinokurov, Vladimir, Andrei Novikov, Valentina Rodnova, Boris Anikushin, Mikhail Kotelev, Evgenii Ivanov, and Yuri Lvov. 2019. "Cellulose Nanofibrils and Tubular Halloysite as Enhanced Strength Gelation Agents" Polymers 11, no. 5: 919. https://doi.org/10.3390/polym11050919
APA StyleVinokurov, V., Novikov, A., Rodnova, V., Anikushin, B., Kotelev, M., Ivanov, E., & Lvov, Y. (2019). Cellulose Nanofibrils and Tubular Halloysite as Enhanced Strength Gelation Agents. Polymers, 11(5), 919. https://doi.org/10.3390/polym11050919