Synthesis of Poly(N-vinylpyrrolidone)-Based Polymer Bottlebrushes by ATRPA and RAFT Polymerization: Toward Drug Delivery Application
Abstract
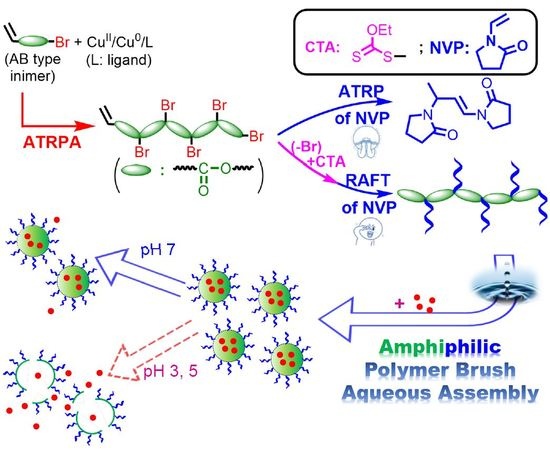
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(4-vinylbenzyl 2-bromo-2-phenylacetate) (PVBBPA) by ATRPA
2.3. Synthesis of Poly(4-vinylbenzyl-2-(ethyl xanthate)-2-phenylacetate) (PVBXPA) Macro-CTA
2.4. Synthesis of PVBPA-g-PNVP Amphiphilic Polymer Bottlebrush by ATRP or RAFT Polymerization
2.5. Encapsulation and Release of Nile Red from PVBPA-g-PNVP Micelles and Tests of MDCK Cell Uptake
2.6. Characterization.
3. Results and Discussion
3.1. Kinetic Study of the Synthesis of Amphiphilic Polymer Bottlebrush
3.2. Micellization and Cell Uptake Study of the Amphiphilic Polymer Bottlebrush
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fierens, S.K.; Telitel, S.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B.; Lutz, J.F.; D’hooge, D.R. Model-based design to push the boundaries of sequence control. Macromolecules 2016, 49, 9336–9344. [Google Scholar] [CrossRef]
- Yokozawa, T.; Ohta, Y. Transformation of step-growth polymerization into living chain-growth polymerization. Chem. Rev. 2016, 116, 1950–1968. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, M. Modern cationic vinyl polymerization. Prog. Polym. Sci. 1991, 16, 111–172. [Google Scholar] [CrossRef]
- Ito, S.; Goseki, R.; Ishizone, T.; Hirao, A. Synthesis of well-controlled graft polymers by living anionic polymerization towards exact graft polymers. Polym. Chem. 2014, 5, 5523–5534. [Google Scholar] [CrossRef]
- Goseki, R.; Ito, S.; Matsuo, Y.; Higashihara, T.; Hirao, A. Precise synthesis of macromolecular architectures by novel iterative methodology combining living anionic polymerization with specially designed linking chemistry. Polymers 2017, 9, 470. [Google Scholar] [CrossRef]
- Huang, C.F.; Aimi, J.; Lai, K.Y. Synthesis of novel mu-star copolymers with poly(N-octyl benzamide) and poly(epsilon-caprolactone) miktoarms through chain-growth condensation polymerization, styrenics-assisted atom transfer radical coupling, and ring-opening polymerization. Macromol. Rapid Commun. 2017, 38, 1600607. [Google Scholar] [CrossRef] [PubMed]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Muller, A.H.E. 50 years of living polymerization. Prog. Polym. Sci. 2006, 31, 1039–1040. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.M.; Derboven, P.; Reyniers, M.F.; Marin, G.B. Model-based design of the polymer microstructure: Bridging the gap between polymer chemistry and engineering. Polym. Chem. 2015, 6, 7081–7096. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B. The strength of multi-scale modeling to unveil the complexity of radical polymerization. Prog. Polym. Sci. 2016, 58, 59–89. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Bian, K.; Cunningham, M.F. Nitroxide-mediated living radical polymerization of 2-hydroxyethyl acrylate and the synthesis of amphiphilic block copolymers. Macromolecules 2005, 38, 695–701. [Google Scholar] [CrossRef]
- Fierens, S.K.; D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B. MAMA-SG1 initiated nitroxide mediated polymerization of styrene: From Arrhenius parameters to model-based design. Chem. Eng. J. 2015, 278, 407–420. [Google Scholar] [CrossRef]
- Moad, G.; Chong, Y.K.; Postma, A.; Rizzardo, E.; Thang, S.H. Advances in RAFT polymerization: The synthesis of polymers with defined end-groups. Polymer 2005, 46, 8458–8468. [Google Scholar] [CrossRef]
- Chen, C.; Guo, X.F.; Du, J.H.; Choi, B.; Tang, H.L.; Feng, A.C.; Thang, S.H. Synthesis of multifunctional miktoarm star polymers via an RGD peptide-based RAFT agent. Polym. Chem. 2019, 10, 228–234. [Google Scholar] [CrossRef]
- De Rybel, N.; Van Steenberge, P.H.M.; Reyniers, M.F.; Barner-Kowollik, C.; D’hooge, D.R.; Marin, G.B. An update on the pivotal role of kinetic modeling for the mechanistic understanding and design of bulk and solution RAFT polymerization. Macromol. Theory Simul. 2017, 26, 1600048. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chen, W.-H.; Aimi, J.; Huang, Y.-S.; Venkatesan, S.; Chiang, Y.-W.; Huang, S.-H.; Kuo, S.-W.; Chen, T. Synthesis of well-defined PCL-b-PnBA-b-PMMA ABC-type triblock copolymers: Toward the construction of nanostructures in epoxy thermosets. Polym. Chem. 2018, 9, 5644–5654. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3745. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Grubbs, R.H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic ring-opening polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Huang, Y.S.; Lai, K.Y. Synthesis and self-assembly of poly(N-octyl benzamide)-μ-poly(ε-caprolactone) miktoarm star copolymers displaying uniform nanofibril morphology. Polymer 2019, 178, 121582. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Varma, I.K. Aliphatic polyesters: Synthesis, properties and applications. Adv. Polym. Sci. 2002, 157, 1–40. [Google Scholar]
- Jerome, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef] [PubMed]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.K. Synthesis of functionalized biodegradable polyesters. Chem. Soc. Rev. 2007, 36, 1573–1580. [Google Scholar] [CrossRef]
- Chen, J.; Huang, S.W.; Liu, M.; Zhuo, R.X. Synthesis and degradation of poly(beta-aminoester) with pendant primary amine. Polymer 2007, 48, 675–681. [Google Scholar] [CrossRef]
- Lynn, D.M.; Langer, R. Degradable poly(beta-amino esters): Synthesis, characterization, and self-assembly with plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- Mather, B.D.; Viswanathan, K.; Miller, K.M.; Long, T.E. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Meng, F.H.; Hennink, W.E.; Zhong, Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef] [PubMed]
- DiCiccio, A.M.; Coates, G.W. Ring-opening copolymerization of maleic anhydride with epoxides: A chain-growth approach to unsaturated polyesters. J. Am. Chem. Soc. 2011, 133, 10724–10727. [Google Scholar] [CrossRef] [PubMed]
- Huijser, S.; HosseiniNejad, E.; Sablong, R.; de Jong, C.; Koning, C.E.; Duchateau, R. Ring-opening co- and terpolymerization of an alicyclic oxirane with carboxylic acid anhydrides and CO2 in the presence of chromium porphyrinato and salen catalysts. Macromolecules 2011, 44, 1132–1139. [Google Scholar] [CrossRef]
- Jeske, R.C.; DiCiccio, A.M.; Coates, G.W. Alternating copolymerization of epoxides and cyclic anhydrides: An improved route to aliphatic polyesters. J. Am. Chem. Soc. 2007, 129, 11330–11331. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.X.; Li, L.; Li, Z.L.; Lv, A.; Du, F.S.; Li, Z.C. Sequence regulated poly(ester-amide)s based on passerini reaction. ACS Macro Lett. 2012, 1, 1300–1303. [Google Scholar] [CrossRef]
- Kreye, O.; Toth, T.; Meier, M.A.R. Introducing multicomponent reactions to polymer science: Passerini reactions of renewable monomers. J. Am. Chem. Soc. 2011, 133, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Solleder, S.C.; Meier, M.A.R. Sequence control in polymer chemistry through the Passerini three-component reaction. Angew. Chem. Int. Ed. 2014, 53, 711–714. [Google Scholar] [CrossRef]
- Ji, S.H.; Bruchmann, B.; Klok, H.A. Exploring the scope of the Baylis-Hillman reaction for the synthesis of side-chain functional polyesters. Macromol. Chem. Phys. 2011, 212, 2612–2618. [Google Scholar] [CrossRef]
- Ji, S.H.; Bruchmann, B.; Klok, H.A. Synthesis of side-chain functional polyesters via Baylis-Hillman polymerization. Macromolecules 2011, 44, 5218–5226. [Google Scholar] [CrossRef]
- Gegenhuber, T.; Schenzel, A.M.; Goldmann, A.S.; Zetterlund, P.B.; Barner-Kowollik, C. A facile route to segmented copolymers by fusing ambient temperature step-growth and RAFT polymerization. Chem. Commun. 2017, 53, 10648–10651. [Google Scholar] [CrossRef]
- Lutz, J.F.; Andrieu, J.; Uzgun, S.; Rudolph, C.; Agarwal, S. Biocompatible, thermoresponsive, and biodegradable: Simple preparation of “all-in-one” biorelevant polymers. Macromolecules 2007, 40, 8540–8543. [Google Scholar] [CrossRef]
- Tran, J.; Pesenti, T.; Cressonnier, J.; Lefay, C.; Gigmes, D.; Guillaneuf, Y.; Nicolas, J. Degradable copolymer nanoparticles from radical ring-opening copolymerization between cyclic ketene acetals and vinyl ethers. Biomacromolecules 2019, 20, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.Y.; Pan, C.Y.; Tang, B.Z. “Living” free radical ring-opening polymerization of 5,6-benzo-2-methylene-1,3-dioxepane using the atom transfer radical polymerization method. Macromolecules 2001, 34, 211–214. [Google Scholar] [CrossRef]
- Wickel, H.; Agarwal, S. Synthesis and characterization of copolymers of 5,6-benzo-2-methylene-1,3-dioxepane and styrene. Macromolecules 2003, 36, 6152–6159. [Google Scholar] [CrossRef]
- Smith, Q.; Huang, J.Y.; Matyjaszewski, K.; Loo, Y.L. Controlled radical polymerization and copolymerization of 5-methylene-2-phenyl-1,3-dioxolan-4-one by ATRP. Macromolecules 2005, 38, 5581–5586. [Google Scholar] [CrossRef]
- Paulusse, J.M.J.; Amir, R.J.; Evans, R.A.; Hawker, C.J. Free radical polymers with tunable and selective bio- and chemical degradability. J. Am. Chem. Soc. 2009, 131, 9805–9812. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-S.; Matyjaszewski, K. Controlled/“living” radical polymerization. Halogen atom transfer radical polymerization promoted by a Cu(I)/Cu(II) redox process. Macromolecules 1995, 28, 7901–7910. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K. Controlled/“living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Matyjaszewski, K. “Green” atom transfer radical polymerization: From process design to preparation of well-defined environmentally friendly polymeric materials. Chem. Rev. 2007, 107, 2270–2299. [Google Scholar] [CrossRef]
- Al-Harthi, M.; Soares, J.B.R.; Simon, L.C. Dynamic Monte Carlo simulation of atom transfer radical polymerization. Macromol. Mater. Eng. 2006, 291, 993–1003. [Google Scholar] [CrossRef]
- Satoh, K.; Mizutani, M.; Kamigaito, M. Metal-catalyzed radical polyaddition as a novel polymer synthetic route. Chem. Commun. 2007, 12, 1260–1262. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.T.; Li, Z.L.; Zhang, L.J.; Du, F.S.; Li, Z.C. Synthesis of linear functionalized polyesters by controlled atom transfer radical polyaddition reactions. Polym. Chem. 2012, 3, 2523–2530. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chou, L.C.; Huang, C.F. Iron-catalysed atom transfer radical polyaddition for the synthesis and modification of novel aliphatic polyesters displaying lower critical solution temperature and pH-dependent release behaviors. Polym. Chem. 2019, 10. [Google Scholar] [CrossRef]
- Satoh, K.; Ozawa, S.; Mizutani, M.; Nagai, K.; Kamigaito, M. Sequence-regulated vinyl copolymers by metal-catalysed step-growth radical polymerization. Nat. Commun. 2010, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.-T.; Dong, Y.-Q.; Du, F.-S.; Li, Z.-C. Controlling polymer topology by atom transfer radical self-condensing vinyl polymerization of p-(2-bromoisobutyloylmethyl)styrene. Macromolecules 2010, 43, 8790–8798. [Google Scholar] [CrossRef]
- Han, Y.-M.; Chen, H.-H.; Huang, C.-F. Polymerization and degradation of aliphatic polyesters synthesized by atom transfer radical polyaddition. Polym. Chem. 2015, 6, 4565–4574. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Lu, L.; Wang, Q.; Benicewicz, B.C. pH and thermal dual-responsive nanoparticles for controlled drug delivery with high loading content. ACS Omega 2017, 2, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Pound, G.; Eksteen, Z.; Pfukwa, R.; McKenzie, J.M.; Lange, R.F.M.; Klumperman, B. Unexpected reactions associated with the xanthate-mediated polymerization of N-vinylpyrrolidone. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6575–6593. [Google Scholar] [CrossRef]
- Huang, C.-F.; Nicolay, R.; Kwak, Y.; Chang, F.-C.; Matyjaszewski, K. Homopolymerization and block copolymerization of N-vinylpyrrolidone by ATRP and RAFT with haloxanthate inifers. Macromolecules 2009, 42, 8198–8210. [Google Scholar] [CrossRef]
- Nicolay, R.; Kwak, Y.; Matyjaszewski, K. Synthesis of poly(vinyl acetate) block copolymers by successive RAFT and ATRP with a bromoxanthate iniferter. Chem. Commun. 2008, 42, 5336–5338. [Google Scholar] [CrossRef]
- Huang, C.-F.; Yoon, J.A.; Matyjaszewski, K. Synthesis of N-vinylcarbazole-N-vinylpyrrolidone amphiphilic block copolymers by xanthate-mediated controlled radical polymerization. Can. J. Chem. 2010, 88, 228–235. [Google Scholar] [CrossRef]









| Sample | n Units of VBBPA | m Units of NVP | Hydrophobic/Hydro-philic Ratio 1 | CMC (mg/mL) 2 | Particle Size (nm) 3 |
|---|---|---|---|---|---|
| (a) | 27 | 4 | 95/5 | 0.39 | 120 |
| (b) | 80 | 120 | 50/50 | 0.28 | 102 |
| (c) | 34 | 60 | 42/58 | 0.49 | 96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-S.; Chen, J.-K.; Kuo, S.-W.; Hsieh, Y.-A.; Yamamoto, S.; Nakanishi, J.; Huang, C.-F. Synthesis of Poly(N-vinylpyrrolidone)-Based Polymer Bottlebrushes by ATRPA and RAFT Polymerization: Toward Drug Delivery Application. Polymers 2019, 11, 1079. https://doi.org/10.3390/polym11061079
Huang Y-S, Chen J-K, Kuo S-W, Hsieh Y-A, Yamamoto S, Nakanishi J, Huang C-F. Synthesis of Poly(N-vinylpyrrolidone)-Based Polymer Bottlebrushes by ATRPA and RAFT Polymerization: Toward Drug Delivery Application. Polymers. 2019; 11(6):1079. https://doi.org/10.3390/polym11061079
Chicago/Turabian StyleHuang, Yi-Shen, Jem-Kun Chen, Shiao-Wei Kuo, Ya-An Hsieh, Shota Yamamoto, Jun Nakanishi, and Chih-Feng Huang. 2019. "Synthesis of Poly(N-vinylpyrrolidone)-Based Polymer Bottlebrushes by ATRPA and RAFT Polymerization: Toward Drug Delivery Application" Polymers 11, no. 6: 1079. https://doi.org/10.3390/polym11061079
APA StyleHuang, Y. -S., Chen, J. -K., Kuo, S. -W., Hsieh, Y. -A., Yamamoto, S., Nakanishi, J., & Huang, C. -F. (2019). Synthesis of Poly(N-vinylpyrrolidone)-Based Polymer Bottlebrushes by ATRPA and RAFT Polymerization: Toward Drug Delivery Application. Polymers, 11(6), 1079. https://doi.org/10.3390/polym11061079