Radiation Degradation of β-Glucan with a Potential for Reduction of Lipids and Glucose in the Blood of Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Degradation of β-glucan
2.3. Determination of Water-Solubility in Irradiated β-glucan Samples
2.4. Mw Determination
2.5. FTIR Measurement
2.6. 1H and 13C-NMR Spectrometry
2.7. X-ray Diffraction
2.8. Experimental Design on Mice
2.9. Blood Plasma Analysis
3. Results and Discussion
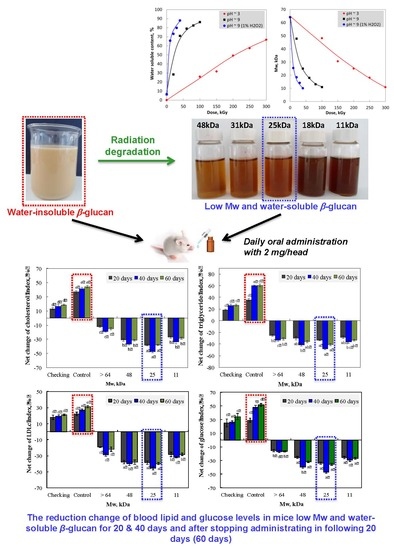
3.1. Change in Mw and Water-Solubility of β-glucan by Irradiation
3.2. Effect of Irradiated β-glucan on the Reduction of the Lipid and Glucose Index in the Blood of Mice
3.2.1. Effect of β-glucan Mw
3.2.2. Effect of Irradiated β-glucan Concentration
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| Da | Dalton |
| GPC | Gel permeation chromatography |
| Gy | Grey |
| FTIR | Fourier transform infrared spectroscopy |
| LDL | low density lipoprotein |
| Mw | molecular weight |
References
- Borchers, A.T.; Stern, J.S.; Hackman, R.M.; Keen, C.L.; Gershwin, M.E. Mushrooms, tumors, and immunity. Proc. Soc. Exp. Biol. Med. 1999, 221, 281–293. [Google Scholar] [PubMed]
- Yamada, H. Bioactive Carbohydrate Polymers; Paulsen, B.S., Ed.; Proceedings of the Phytochemical Society of Europe; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2000; Volume 44. [Google Scholar]
- Brow, G.D.; Gordon, S. Fungal beta-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Zhu, F.M.; Du, B.; Bian, Z.X.; Xu, B.J. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Mantovani, S.M.; Bellini, F.M.; Angeli, F.J.; Oliveira, R.; Silva, F.A.; Ribeiro, R.L. β-Glucans in promoting health: Prevention against mutation and cancer. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.; Nagodawithana, T.W. Yeast-derived products and food and feed yeast. In Yeast Technology; Van Nostrand Reinhold: New York, NY, USA, 1991. [Google Scholar]
- Zechner-krpan, V.; Petravić-tominac, V.; Panjkota-krbavčić, I.; Grba, S.; Berković, K. Potential application of yeast β-Glucans in food industry. Agric. Conspec. Sci. 2009, 74, 277–282. [Google Scholar]
- Stokes, C.R.; Miller, B.G.; Bailey, M.; Wilson, A.D.; Bourne, F.J. The immune response to dietary antigens and its influence on disease susceptibility in farm animals. Vet. Immunol. Immunopathol. 1987, 17, 413–423. [Google Scholar] [CrossRef]
- Suzuki, T.; Tanaka, H.; Kinoshita, A.; Oikawa, S.; Osawa, M.; Yadomae, T. Effect of orally administered beta-glucan in macrophage function in mice. Int. J. Immunopharmacol. 1990, 12, 675–684. [Google Scholar] [CrossRef]
- Dritz, S.S.; Shi, J.; Kielian, T.L.; Goodband, R.D.; Nelsen, J.L.; Tokach, M.D.; Chengappa, M.M.; Smith, J.E.; Blecha, F. Influence of dietary β-glucan on growth performance, nonspecific immunity and resistance to Streptococcus suis infection in weanling pigs. J. Anim. Sci. 1995, 73, 3341–3350. [Google Scholar] [CrossRef]
- Chae, B.J.; Lohakare, J.D.; Moon, W.K.; Lee, S.L.; Park, Y.H.; Hahn, T.W. Effects of supplementation of β-glucan on the growth performance and immunity in broilers. Res. Vet. Sci. 2006, 80, 291–298. [Google Scholar] [CrossRef]
- Liepins, J.; Kovačova, E.; Shivirksts, K.; Grube, M.; Rapoport, A.; Kogan, G. Drying enhances immunoactivity of spent brewer’s yeast cell wall β-D-glucans. J. Biotechnol. 2015, 206, 12–16. [Google Scholar] [CrossRef]
- Johnson, I.T.; Gee, J.M. Effect of gel-forming gums on the intestinal unstirred layer and sugar transport in vitro. Gut 1981, 22, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Axelsen, M.; Augustin, L.S.; Vuksan, V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr. Opin. Lipidol. 2000, 11, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sung, N.Y.; Choi, J.; Yoon, Y.; Lee, S.Y.; Byun, M.W.; Hwang, Y.J.; Koenari, Z.I.; Lee, J.W.; Kim, J.H. Anti-allergic Effect of Low Molecular Weight β-Glucan Prepared by γ-Irradiation. Food Sci. Biotechnol. 2011, 20, 841–844. [Google Scholar] [CrossRef]
- Lee, J.W.; Byun, E.H.; Sung, N.Y.; Raghavendran, H.R.B.; Byun, E.B.; Kim, J.H.; Choi, J.; Shin, M.G.; Byun, M.W. Effect of gamma irradiation on the efficacy of β-glucan against acetaminophen induced toxicity in mice. Chem. Biol. Interact. 2009, 180, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Sima, P.; Vannucci, L.; Vetvicka, V. β-glucans and cholesterol (Review). Int. J. Mol. Med. 2018, 41, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Zlatkovic, D.; Jakovljevic, D.; Zekovic, D.; Miroslav, M.V. A glucan from active dry baker’s yeast (Saccharomyces cerevisiae): A chemical and enzymatic investigation of the structure. J. Serb. Chem. Soc. 2003, 68, 805–809. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. Microbiol. Rev. 2002, 26, 239–256. [Google Scholar]
- Sung, N.Y.; Byun, E.H.; Kwon, S.K.; Song, B.S.; Choi, J.I.; Kim, J.H.; Byun, M.W.; Yoo, Y.C.; Kim, M.R.; Lee, J.W. Immune enhancing activities of low molecular weight β-glucan depolymerized by gamma irradiation. Radiat. Phys. Chem. 2009, 78, 433–436. [Google Scholar] [CrossRef]
- Methacanon, P.; Weerawatsophon, U.; Tanjak, P.; Rachtawee, P.; Prathumpai, W. Interleukin-8 stimulating activity of low molecular weight β-glucan depolymerized by γ-irradiation. Carbohydr. Polym. 2011, 86, 574–580. [Google Scholar] [CrossRef]
- Hasegawa, M.; Isogai, A.; Onabe, F. Preparation of low molecular weight chitosan using phosphoric acid. Carbohydr. Polym. 1993, 20, 279–283. [Google Scholar] [CrossRef]
- Bohn, J.A.; BeMiller, J.N. (1-3)-β-d-glucans-d-glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Shimokawa, T.; Yoshida, S.; Takeuchi, T.; Murata, K.; Ishii, T.; Kusakabe, I. Preparation of two series of oligoguluronic acids from sodium alginate by acid hydrolysis and enzymatic degradation. Biosci. Biotechnol. Biochem. 1996, 60, 1532–1534. [Google Scholar] [CrossRef]
- Ilyina, A.V.; Tikhonov, V.E.; Albulov, A.I.; Varlamov, V.P. Enzymic preparation of acid-free-water-soluble chitosan. Process Biochem. 2000, 35, 563–568. [Google Scholar] [CrossRef]
- Sandula, J.; Kogan, G.; Kacurakova, M.; Machova, E. Microbial (1-3)-β-d-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohydr. Polym. 1999, 3, 247–253. [Google Scholar] [CrossRef]
- Roubroeks, J.P.; Andersson, R.; Mastromauro, D.I.; Christensen, B.E.; Åman, P. Molecular weight, structure and shape of oat (1-3),(1-4)-β-d-glucan fractions obtained by enzymatic degradation with (1-4)-β-d-glucan 4-glucanohydrolase from Trichoderma reesei. Carbohydr. Polym. 2001, 46, 275–285. [Google Scholar] [CrossRef]
- Shin, H.J.; Lee, D.C. Study on the process to decrease the molecular weight of beta-(1,6)-branched beta-(1,3)-D-glucans. Korean Soc. Biotechnol. Bioeng. 2003, 18, 352–355. [Google Scholar]
- Moradali, M.F.; Mostafavi, H.; Ghods, S.; Hedjaroude, G.A. Immunomodulation and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunol. 2007, 7, 701–724. [Google Scholar]
- Marie, C.R.; Monique, A.V.A.; Jean, F.T. Gelation properties of extruded lemon cell walls and their water-soluble pectins. Carbohydr. Res. 1994, 26, 271–282. [Google Scholar]
- Jeon, Y.I.; Kim, S.K. Production of chito-oligosaccharides using an ultrafiltration membrane reactor and their antibacterial activity. Carbohydr. Res. 2002, 41, 133–141. [Google Scholar] [CrossRef]
- Charlesby, A. Crosslinking and degradation of polymers. Radiat. Phys. Chem. 1981, 18, 59–66. [Google Scholar] [CrossRef]
- Luan, L.Q.; Ha, V.T.T.; Nagasawa, N.; Kume, T.; Yoshii, F.; Nakanishi, T.M. Biological effect of irradiated chitosan on plants in vitro. Biotechnol. Appl. Biochem. 2005, 41, 49–57. [Google Scholar]
- Duy, N.N.; Phu, D.V.; Anh, N.T.; Hien, N.Q. Synergistic degradation to prepare oligochitosan by γ-irradiation of chitosan solution in the presence of hydrogen peroxide. Radiat. Phys. Chem. 2011, 80, 848–853. [Google Scholar] [CrossRef]
- Luan, L.Q.; Ha, V.T.T.; Uyen, N.H.P.; Trang, L.T.T.; Hien, N.Q. Preparation of oligoalginate plant growth promoter by γ irradiation of alginate solution containing hydrogen peroxide. J. Agric. Food Chem. 2012, 60, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- Dung, P.D.; Hung, L.T.; Ha, T.L.T.; Luan, L.Q.; Le, B.V.; Thang, N.T. Study on the biological effects of oligochitosan fractions, prepared by synergistic degradation method, on capsicum. Int. J. Polym. Sci. 2018, 2018. [Google Scholar] [CrossRef]
- Sokhey, A.S.; Hanna, M.A. Properties of irradiated starches. Food Struct. 1993, 12, 397–410. [Google Scholar]
- Williams, D.L.; Pretus, H.A.; McNamee, R.B.; Jones, E.L.; Ensley, H.E.; Browder, I.W. Development of a water-soluble, sulfated (1-3)-β-d-glucan biological response modifier derived from Saccharomyces cerevisiae. Carbohydr. Res. 1992, 235, 247–257. [Google Scholar] [CrossRef]
- Byun, E.H.; Kim, J.H.; Sung, N.Y.; Choi, J.I.; Lim, S.T.; Kim, K.H.; Yook, H.S.; Byun, M.W.; Lee, J.W. Effects of gamma irradiation on the physical and structural properties of β-glucan. Radiat. Phys. Chem. 2008, 77, 781–786. [Google Scholar] [CrossRef]
- Williams, D.L.; McNamee, R.B.; Jones, E.L.; Pretus, H.A.; Ensley, H.E.; Browder, I.W.; Di Luzio, N.R. A method for the solubilization of a (1-3)-β-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr. Res. 1991, 219, 203–213. [Google Scholar] [CrossRef]
- Ershos, B.G. Radiation-chemical degradation of cellulose and other polysaccharides. Russ. Chem. Rev. 1998, 67, 315–334. [Google Scholar] [CrossRef]
- Copikova, J.; Synysya, A.; Cerna, M.; Kaasova, J.; Novotna, M. Application of FT-IR spectroscopy in detection of food hydrocolloids in confectionery jellies and food supplements. Czech J. Food Sci. 2001, 19, 51–56. [Google Scholar] [CrossRef]
- Charlesby, A. Atomic Radiation and Polymer; Pergamon Press Ltd.: New York, NY, USA, 1960. [Google Scholar]
- Ulanski, P.; Rosiak, J. Preliminary studies on radiation-induced changes in chitosan. Radiat. Phys. Chem. 1992, 39, 53–57. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, E.H.; Cheong, C.; Williams, D.L.; Kim, C.W.; Lim, S.T. Structural characterization of beta-D-(1→3, 1→6)-linked glucans using NMR spectroscopy. Carbohydr. Res. 2000, 328, 331–341. [Google Scholar] [CrossRef]
- Du, L.; Zhang, X.; Wang, C.; Xiao, D. Preparation of water-soluble yeast glucan by four kinds of solubilizing process. Engineering 2012, 5, 184–188. [Google Scholar] [CrossRef]
- Kim, J.H.; Sung, N.Y.; Byun, E.H.; Kwon, S.K.; Song, B.S.; Choi, J.; Yoon, Y.; Kim, J.K.; Byun, M.W.; Lee, J.W. Effects of γ-irradiation on immunological activities of β-glucan. Food Sci. Biotechnol. 2009, 18, 1305–1309. [Google Scholar]
- Waszkiewicz-Robak, B. Spent Brewer’s Yeast and Beta-Glucans Isolated from Them as Diet Components Modifying Blood Lipid Metabolism Disturbed by an Atherogenic Diet; Lipid Metabolism; Intech: Dundas, ON, Canada, 2013; pp. 262–290. [Google Scholar]
- U.S. Food and Drug Administration. FDA final rule for federal labeling: Health claims; oats and coronary heart disease. Fed. Regist. 1997, 62, 3584–3681. [Google Scholar]
- Talati, R.; Baker, W.L.; Pabilonia, M.S.; Whi, C.M.; Coleman, C.I. The effects of barley-derived soluble fiber on serum lipids. Ann. Fam. Med. 2009, 7, 157–163. [Google Scholar] [CrossRef] [PubMed]
- AbuMweis, S.S.; Jew, S.; Ames, N.P. β-glucan from barley and its lipid-lowering capacity: A meta-analysis of randomized, controlled trials. Eur. J. Clin. Nutr. 2010, 64, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. A comparison of injected and orally administered β-glucans. J. Am. Nutraceutical Assoc. 2008, 11, 1–8. [Google Scholar]
- Gidley, M.J. Hydrocolloids in the digestive tract and related health implications. Curr. Opin. Colloid Int. Sci. 2013, 18, 371–378. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Theuwissen, E.; Mensink, R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 2008, 94, 285–292. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, N.T.; Anh, N.T.N.; Giang, B.L.; Son, H.N.; Luan, L.Q. Radiation Degradation of β-Glucan with a Potential for Reduction of Lipids and Glucose in the Blood of Mice. Polymers 2019, 11, 955. https://doi.org/10.3390/polym11060955
Long NT, Anh NTN, Giang BL, Son HN, Luan LQ. Radiation Degradation of β-Glucan with a Potential for Reduction of Lipids and Glucose in the Blood of Mice. Polymers. 2019; 11(6):955. https://doi.org/10.3390/polym11060955
Chicago/Turabian StyleLong, Nguyen Thanh, Nguyen Thi Ngoc Anh, Bach Long Giang, Hoang Nghia Son, and Le Quang Luan. 2019. "Radiation Degradation of β-Glucan with a Potential for Reduction of Lipids and Glucose in the Blood of Mice" Polymers 11, no. 6: 955. https://doi.org/10.3390/polym11060955
APA StyleLong, N. T., Anh, N. T. N., Giang, B. L., Son, H. N., & Luan, L. Q. (2019). Radiation Degradation of β-Glucan with a Potential for Reduction of Lipids and Glucose in the Blood of Mice. Polymers, 11(6), 955. https://doi.org/10.3390/polym11060955