Formation Features of Hybrid Nanocomposites Based on Polydiphenylamine-2-Carboxylic Acid and Single-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of SWCNT/PDPAC Nanocomposites
2.3. Synthesis of PDPAC
2.4. Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of Materials


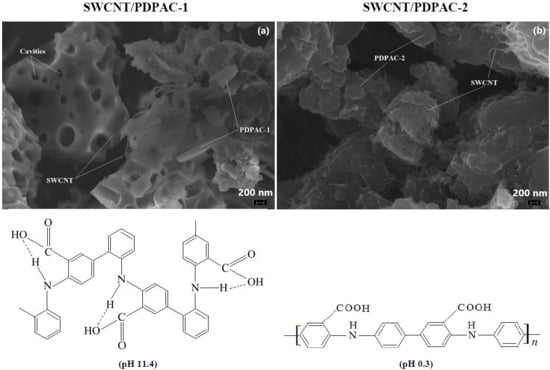
3.2. Morphology of Nanocomposites
3.3. Thermal Properties of Materials
3.4. Electrical Characterization of Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Hamon, M.A.; Chen, J.; Hu, H.; Chen, Y.; Itkis, M.E.; Rao, A.M.; Eklund, P.C.; Haddon, R. Dissolution of single-walled carbon nanotubes. Adv. Mater. 1999, 11, 834–840. [Google Scholar] [CrossRef]
- Riggs, J.E.; Guo, Z.; Carroll, D.L.; Sun, Y.-P. Strong luminescence of solubilized carbon nanotubes. J. Am. Chem. Soc. 2000, 122, 5879–5880. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Mau, A.V.H. Controlled synthesis and modification of carbon nanotubes and C60: Carbon nanostructures for advanced polymeric composite materials. Adv. Mater. 2001, 13, 899–913. [Google Scholar] [CrossRef]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance properties of poly(3,4-ethylene- dioxythiophene)/carbon nanotubes composites. J. Phys. Chem. Solids 2004, 65, 295–301. [Google Scholar] [CrossRef]
- Wu, T.M.; Lin, Y.W.; Liao, C.S. Preparation and characterization of polyaniline/multiwalled carbon nanotube composites. Carbon 2005, 43, 734–740. [Google Scholar] [CrossRef]
- Wu, T.M.; Lin, Y.W. Synthesis, characterization, and electrical properties of polypyrrole/multiwalled carbon nanotube composites. J. Polym. Sci. Polym. Chem. 2006, 44, 6449–6457. [Google Scholar] [CrossRef]
- Li, C.; Bai, H.; Shi, G. Conducting polymer nanomaterials: Electrosynthesis and applications. Chem. Soc. Rev. 2009, 38, 2397–2409. [Google Scholar] [CrossRef]
- Sapurina, I.; Stejskal, J. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polym. Int. 2008, 57, 1295–1325. [Google Scholar] [CrossRef]
- Sapurina, I.Y.; Stejskal, J. The effect of pH on the oxidative polymerization of aniline and the morphology and properties of products. Russ. Chem. Rev. 2010, 79, 1123–1143. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Oueiny, C.; Berlioz, S.; Perrin, F.-X. Carbon nanotube–polyaniline composites. Prog. Polym. Sci. 2014, 39, 707–748. [Google Scholar] [CrossRef]
- Luo, J.; Niu, H.-J.; Wu, W.-J.; Wang, C.; Bai, X.-D.; Wang, W. Enhancement of the efficiency of dye-sensitized solar cell with multi-wall carbon nanotubes/polythiophene composite counter electrodes prepared by electrodeposition. Solid State Sci. 2012, 14, 145–149. [Google Scholar] [CrossRef]
- Gao, M.; Huang, S.M.; Dai, L.M.; Wallace, G.; Gao, R.P.; Wang, Z.L. Aligned coaxial nanowires of carbon nanotubes sheathed with conducting polymers. Angew. Chem. Int. Ed. 2000, 39, 3664–3667. [Google Scholar] [CrossRef]
- Hughes, M.; Shaffer, M.S.P.; Renouf, A.C.; Singh, C.; Chen, G.Z.; Fray, J.; Windle, A.H. Electrochemical capacitance of nanocomposite films formed by coating aligned arrays of carbon nanotubes with polypyrrole. Adv. Mater. 2002, 14, 382–385. [Google Scholar] [CrossRef]
- Chen, G.Z.; Shaffer, M.S.P.; Coleby, D.; Dixon, G.; Zhou, W.Z.; Fray, D.J.; Windle, A.H. Carbon nanotube and polypyrrole composites: Coating and doping. Adv. Mater. 2000, 12, 522–526. [Google Scholar] [CrossRef]
- Wu, M.Q.; Snook, G.A.; Gupta, V.; Shaffer, M.; Fray, D.J.; Chen, G.Z. Electrochemical fabrication and capacitance of composite films of carbon nanotubes and polyaniline. J. Mater. Chem. 2005, 15, 2297–2303. [Google Scholar] [CrossRef]
- Peng, C.; Snook, G.A.; Fray, D.J.; Shaffer, M.S.P.; Chen, G.Z. Carbon nanotube stabilised emulsions for electrochemical synthesis of porous nanocomposite coatings of poly[3,4-ethylene-dioxythiophene]. Chem. Commun. 2006, 44, 4629–4631. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Asanov, I.P.; Galkin, P.S.; Bulusheva, L.G.; Chekhova, G.N.; Kurenya, A.G.; Shubin, Y.V. Composites based on polyaniline and aligned carbon nanotubes. Polym. Sci. B 2010, 52, 101–108. [Google Scholar] [CrossRef]
- Jin, L.; Bowe, C.; Zhou, O. Alignment of carbon nanotubes in a polymer matrix by mechanical stretching. Appl. Phys. Lett. 1998, 73, 1197–1199. [Google Scholar] [CrossRef]
- Zengin, H.; Zhou, W.; Jin, J.; Czerw, R.; Smith, D.W., Jr.; Echegoyen, L.; Carroll, D.L.; Foulger, S.H.; Ballato, J. Carbon nanotube doped polyaniline. Adv. Mater. 2002, 14, 1480–1483. [Google Scholar] [CrossRef]
- Gerasin, V.A.; Antipov, E.M.; Karbushev, V.V.; Kulichikhin, V.G.; Karpacheva, G.P.; Talroze, R.V.; Kudryavtsev, Y.V. New approaches to the development of hybrid nanocomposites: From structural materials to high-tech applications. Russ. Chem. Rev. 2013, 82, 303–332. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Motlagh, G.H.; Garakani, S.S.; Boroomand, A. Effects of multiwall carbon nanotubes on the polymerization model of aniline. J. Polym. Res. 2018, 25, 265. [Google Scholar] [CrossRef]
- Ham, H.T.; Choi, Y.S.; Jeong, N.; Chung, I.J. Single-wall carbon nanotubes covered with polypyrrole nanoparticles by the miniemulsion polymerization. Polymer 2005, 46, 6308–6315. [Google Scholar] [CrossRef]
- Park, Ch.; Ounaies, Z.; Watson, K.A.; Crooks, R.E.; Smith, J., Jr.; Lowther, S.E.; Connell, J.W.; Siochi, E.J.; Harrison, J.S.; Terry, L.S. Dispersion of single wall carbon nanotubes by in situ polymerization under sonication. Chem. Phys. Lett. 2002, 364, 303–308. [Google Scholar]
- Jeevananda, T.; Siddaramaiah, D.; Lee, T.S.; Lee, J.H.; Samir, O.M.; Somashekar, R. Polyaniline-multiwalled carbon nanotube composites: Characterization by WAXS and TGA. J. Appl. Polym. Sci. 2008, 109, 200–210. [Google Scholar] [CrossRef]
- Zelikman, E.; Narkis, M.; Siegmann, A.; Valentini, L.; Kenny, J.M. Polyaniline/multiwalled carbon nanotube systems: Dispersion of CNT and CNT/PANI interaction. Polym. Eng. Sci. 2008, 48, 1871–1877. [Google Scholar] [CrossRef]
- Konyushenko, E.N.; Stejskal, J.; Trchova, M.; Hradil, J.; Kovarova, J.; Prokes, J.; Cieslar, M.; Hwang, J.-Y.; Chen, K.-H.; Sapurina, I. Multi-wall carbon nanotubes coated with polyaniline. Polymer 2006, 47, 5715–5723. [Google Scholar] [CrossRef]
- Ginic-Markovic, M.; Matisons, J.G.; Cervini, R.; Simon, G.P.; Fredericks, P.M. Synthesis of new polyaniline/nanotube composites using ultrasonically initiated emulsion polymerization. Chem. Mater. 2006, 18, 6258–6265. [Google Scholar] [CrossRef]
- Suckeveriene, R.Y.; Zelikman, E.; Mechrez, G.; Tzur, A.; Frisman, I.; Cohen, Y.; Narkis, M. Synthesis of hybrid polyaniline/carbon nanotube nanocomposites by dynamic interfacial inverse emulsion polymerization under sonication. J. Appl. Polym. Sci. 2011, 120, 676–682. [Google Scholar] [CrossRef]
- Zelikman, E.; Suckeveriene, R.Y.; Mechrez, G.; Narkis, M. Fabrication of composite polyaniline/CNT nanofiber/s using an ultrasonically assisted dynamic inverse emulsion polymerization technique. Polym. Adv. Technol. 2010, 21, 150–152. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Liu, Z. Tubular composite of doped polyaniline with multi-walled carbon nanotubes. Appl. Phys. A Mater. Sci. Process. 2004, 80, 1813–1817. [Google Scholar] [CrossRef]
- Sainz, R.; Benito, A.M.; Martínez, M.T.; Galindo, J.F.; Sotres, J.; Baró, A.M.; Corraze, B.; Chauvet, O.; Maser, W.K. Soluble self-aligned carbon/polyaniline composites. Adv. Mater. 2005, 17, 278–281. [Google Scholar] [CrossRef]
- Cochet, M.; Maser, W.K.; Benito, A.M.; Callejas, M.A.; Martínez, M.T.; Benoit, J.-M.; Schreiber, J.; Chauvet, O. Synthesis of a new polyaniline/nanotube composite: “in-situ” polymerization and charge transfer through site-selective interaction. Chem. Commun. 2001, 37, 1450–1451. [Google Scholar] [CrossRef]
- Deng, M.; Yang, B.; Hu, Y. Polyaniline deposition to enhance the specific capacitance of carbon nanotubes for supercapacitors. J. Mater. Sci. 2005, 40, 5021–5023. [Google Scholar] [CrossRef]
- Yu, Y.; Che, B.; Si, Zh.; Li, L.; Chen, W.; Xue, G. Carbon nanotube/polyaniline core-shell nanowires prepared by in situ inverse microemulsion. Synth. Met. 2005, 150, 271–277. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Islam, A.; Zia, S.; Shafiq, M.; Sabir, A.; Munawar, M.A.; Butt, M.T.Z.; Jamil, T. Effect of different oxidants on polyaniline/single walled carbon nanotubes composites synthesized via ultrasonically initiated in-situ chemical polymerization. Mater. Chem. Phys. 2016, 172, 39–46. [Google Scholar] [CrossRef]
- Dhand, C.; Solanki, P.R.; Datta, M.; Malhotra, B.D. Polyaniline/single-walled carbon nanotubes composite based triglyceride biosensor. Electroanalysis 2010, 22, 2683–2693. [Google Scholar] [CrossRef]
- Qu, F.; Yang, M.; Jiang, J.; Shen, G.; Yu, R. Amperometric biosensor for choline based on layer-by-layer assembled functionalized carbon nanotube and polyaniline multilayer film. Anal. Biochem. 2005, 344, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, H.; Yu, A.; Perea, D.; Haddon, R.C. Synthesis and characterization of water soluble single-walled carbon nanotube graft copolymers. J. Am.Chem. Soc. 2005, 127, 8197–8203. [Google Scholar] [CrossRef] [PubMed]
- Philip, B.; Xie, J.; Abraham, J.K.; Varadan, V.K. Polyaniline/carbon nanotube composites: Starting with phenylamino functionalized carbon nanotubes. Polym. Bull. 2005, 53, 127–138. [Google Scholar] [CrossRef]
- Xu, J.; Yao, P.; Liu, L.; Jiang, Zh.; He, F.; Li, M.; Zou, J. Synthesis and characterization of an organic soluble and conducting polyaniline-grafted multiwalled carbon nanotube core–shell nanocomposites by emulsion polymerization. J. Appl. Polym. Sci. 2010, 118, 2582–2591. [Google Scholar] [CrossRef]
- Kar, P.; Choudhury, A. Carboxylic acid functionalized multi-walled carbon nanotube doped polyaniline for chloroform sensors. Sens. Actuators B Chem. 2013, 183, 25–33. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Wasfi, A.S. A comparative study of polyaniline/MWCNT with polyaniline/SWCNT nanocomposite films synthesized by microwave plasma polymerization. Synth. Met. 2019, 250, 49–54. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Karpacheva, G.P. Hybrid material based on poly-3-amine-7-methylamine-2-methylphenazine and single-walled carbon nanotubes and method of its production. Polymers 2018, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.Z.; Karpacheva, G.P.; Kolyagin, Yu.G. Hybrid nanocomposite based on poly-3-amine-7-methylamine-2-methylphenazine and single-walled carbon nanotubes. Polym. Bull. 2018. [Google Scholar] [CrossRef]
- Ozkan, S.Zh.; Eremeev, I.S.; Karpacheva, G.P.; Bondarenko, G.N. Oxidative polymerization of N-phenylanthranilic acid in the heterophase system. Open, J. Polym. Chem. 2013, 3, 63–69. [Google Scholar] [CrossRef]
- Ozkan, S.Zh.; Bondarenko, G.N.; Karpacheva, G.P. Oxidative polymerization of diphenylamine-2-carboxylic acid: Synthesis, structure, and properties of polymers. Polym. Sci. B 2010, 52, 263–269. [Google Scholar] [CrossRef]
- Ozkan, S.Zh.; Eremeev, I.S.; Karpacheva, G.P.; Prudskova, T.N.; Veselova, E.V.; Bondarenko, G.N.; Shandryuk, G.A. Polymers of dipheylamine-2-carboxylic acid: Synthesis, structure and properties. Polym. Sci. B 2013, 55, 107–115. [Google Scholar]
- Karyakin, Yu.V.; Angelov, I.I. Pure Chemical Reagents; Khimiya: Moscow, Russia, 1974. (In Russian) [Google Scholar]
- Thakur, R.S.; Kurur, N.D.; Madhu, P.K. Swept-frequency two-pulse phase modulation for heteronuclear dipolar decoupling in solid-state NMR. Chem. Phys. Lett. 2006, 426, 459–463. [Google Scholar] [CrossRef]
- Earl, W.L.; Vanderhart, D.L. Measurement of 13C chemical shifts in solids. J. Magn. Reson. 1982, 48, 35–54. [Google Scholar] [CrossRef]
- Morcombe, C.R.; Zilm, K.W. Chemical shift referencing in MAS solid state NMR. J. Magn. Reson. 2003, 162, 479–486. [Google Scholar] [CrossRef]
- Peng, H.; Alemany, L.B.; Margrave, J.L.; Khabashesku, V.N. Sidewall carboxylic acid functionalization of single-walled carbon nanotubes. J. Am. Chem. Soc. 2003, 125, 15174–15182. [Google Scholar] [CrossRef] [PubMed]
- Eletskii, A.V.; Knizhnik, A.A.; Potapkin, B.V.; Kenny, J.M. Electrical characteristics of carbon nanotube doped composites. Uspekhi Phyzicheskikh Nauk. 2015, 185, 225–270. [Google Scholar] [CrossRef] [Green Version]
- Rehwald, W.; Kiess, H.; Binggeli, B. Frequency dependent conductivity in polymers and other disordered materials. Z. Phys. B Condens. Matter. 1987, 68, 143–148. [Google Scholar] [CrossRef]
- Dyre, J.C. The random free-energy barrier model for ac conduction in disordered solids. J. Appl. Phys. 1988, 64, 2456–2468. [Google Scholar] [CrossRef]
- Coleman, J.N.; Curran, S.; Dalton, A.B.; Davey, A.P.; McCarthy, B.; Blau, W.; Barklie, R.C. Percolation-dominated conductivity in a conjugated-polymer-carbon-nanotube composite. Phys. Rev. B 1998, 58, R7492(R)–R7495(R). [Google Scholar] [CrossRef]
- Potphode, D.D.; Sivaraman, P.; Mishra, S.P.; Patri, M. Polyaniline/partially exfoliated multi-walled carbon nanotubes based nanocomposites for supercapacitors. Electrochim. Acta 2015, 155, 402–410. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.-F.; Hou, H. Electrospun carbon nanofibers surface-grown with carbon nanotubes and polyaniline for use as high-performance electrode materials of supercapacitors. RSC Adv. 2014, 4, 23622–23629. [Google Scholar] [CrossRef]
- Ha, J.-S.; Lee, J.-M.; Lee, H.-R.; Huh, P.; Jo, N.-J. Polymer nanocomposite electrode consisting of polyaniline and modified multi-walled carbon nanotube for rechargeable battery. J. Nanosci. Nanotechnol. 2015, 15, 8977–8983. [Google Scholar] [CrossRef]
- He, B.L.; Dong, B.; Wang, W.; Li, H.L. Performance of polyaniline/multi-walled carbon nanotubes composites as cathode for rechargeable lithium batteries. Mater. Chem. Phys. 2009, 114, 371–375. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Kale, B.B. Studies of conducting polyaniline (PANI) wrapped-multiwalled carbon nanotubes (MWCNTs) nanocomposite and its application for optical pH sensing. Sens. Actuators B Chem. 2013, 187, 407–412. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Sadhukhan, P.; Naskar, K.; Lal, G.; Bhar, R.; Sinha, C.; Das, S. Polyaniline-multiwalled carbon nanotube (PANI-MWCNT): Room temperature resistive carbon monoxide (CO) sensor. Synth. Met. 2018, 245, 182–189. [Google Scholar] [CrossRef]
- Bachnav, S.G.; Patil, D.R. Study of polypyrrole-coated MWCNT nanocomposites for ammonia sensing at room temperature. J. Mater. Sci. Chem. Eng. 2015, 3, 30–44. [Google Scholar]
- Xue, L.; Wang, W.; Guo, Y.; Wan, P. Flexible polyaniline/carbon nanotube nanocomposite film-based electronic gas sensors. Sens. Actuators B Chem. 2017, 244, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Dutra, F.V.A.; Pires, B.C.; Nascimento, T.A.; Borges, K.B. Functional polyaniline/multiwalled carbon nanotube composite as an efficient adsorbent material for removing pharmaceuticals from aqueous media. J. Environ. Manag. 2018, 221, 28–37. [Google Scholar] [CrossRef]
- Jin, Y.W.; Jung, J.E.; Park, Y.J.; Choi, J.H.; Jung, D.S.; Lee, H.W.; Park, S.H.; Lee, N.S.; Kim, J.M.; Ko, T.Y.; et al. Triode-type field emission array using carbon nanotubes and a conducting polymer composite prepared by electrochemical polymerization. J. Appl. Phys. 2002, 92, 1065–1069. [Google Scholar] [CrossRef]
- Deshpande, P.; Vathare, S.; Vagge, S.; Tomšík, E.; Stejskal, J. Conducting polyaniline/multi-wall carbon nanotubes composite paints on low carbon steel for corrosion protection: Electrochemical investigations. Chem. Pap. 2013, 67, 1072–1078. [Google Scholar] [CrossRef]
- Xiao, Y.; Lin, J.-Y.; Wu, J.; Tai, S.-Y.; Yue, G.; Lin, T.-W. Dye-sensitized solar cells with high-performance polyaniline/multi-wall carbon nanotube counter electrodes electropolymerized by a pulse potentiostatic technique. J. Power Sources 2013, 233, 320–325. [Google Scholar] [CrossRef]
- He, B.; Tang, Q.; Liang, T.; Li, Q. Efficient dye-sensitized solar cells from polyaniline-single wall carbon nanotube complex counter electrodes. J. Mater. Chem. A 2014, 2, 3119–3126. [Google Scholar] [CrossRef]
- Saranya, K.; Rameez, M.; Subramania, A. Developments in conducting polymer based counter electrodes for dye-sensitized solar cells—An overview. Eur. Polym. J. 2015, 66, 207–227. [Google Scholar] [CrossRef]
- Peng, S.; Wu, Y.; Zhu, P.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Facile fabrication of polypyrrole/functionalized multiwalled carbon nanotubes composite as counter electrodes in low-cost dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2011, 223, 97–102. [Google Scholar] [CrossRef]
- Abdul Almohsin, S.; AL-Mutoki, S.M.; Li, Z. Electrochemical polymerization of PPy–MWCNT composite as a counter electrode for dye-sensitized solar cells. J. Arkansas Acad. Sci. 2012, 66, 31–35. [Google Scholar]
- He, B.; Tang, Q.; Luo, J.; Li, Q.; Chen, X.; Cai, H. Rapid charge-transfer in polypyrrole–single wall carbon nanotube complex counter electrodes: Improved photovoltaic performances of dye-sensitized solar cells. J. Power Sources 2014, 256, 170–177. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Ting, P.-N.; Luo, S.-H.; Lin, J.-Y. Pulse-reversal electropolymerization of polypyrrole on functionalized carbon nanotubes as composite counter electrodes in dye-sensitized solar cells. Electrochim. Acta 2014, 137, 721–727. [Google Scholar] [CrossRef]
- Niu, H.; Qin, S.; Mao, X.; Zhang, S.; Wang, R.; Wan, L.; Xua, J.; Miao, S. Axle-sleeve structured MWCNTs/polyaniline composite film as cost-effective counter-electrodes for high efficient dye-sensitized solar cells. Electrochim. Acta 2014, 121, 285–293. [Google Scholar] [CrossRef]









| Assignment of Absorption Bands | Frequency ν, cm−1 | ||||
|---|---|---|---|---|---|
| PDPAC-1 | SWCNT/PDPAC-1 | PDPAC-2 | SWCNT/PDPAC-2 | DPAC (monomer) | |
| Stretching vibrations of vN–H | 3236 | 3175 | 3303 | 3308 | 3337 |
| H—N-H with hydrogen bond | 3293 | 3264 | - | - | - |
| Stretching vibrations of vC–H in an aromatic ring | 3081 | 3062 | 3020 | 3033 | 3034 |
| Stretching vibrations of vC=O in COOH | 1682 1231 | 1680 1215 | 1659 1226 | 1655 1218 | 1658 1259 |
| Stretching vibrations of vC–C in an aromatic ring | 1595 1509 | 1585 1495 | 1584 1500 | 1582 1498 | 1575 1509 |
| Stretching vibrations of vC–N | 1316 | 1313 | 1311 | 1310 | 1323 |
| Out-of-plane bending vibrations of δC–H in an 1,2-substituted aromatic ring | 753 | 748 | 751 | 746 | 746 |
| Out-of-plane bending vibrations of δC–H in an 1,2,4-trisubstituted aromatic ring | 828 | 828 | 803 | 802 | - |
| Out-of-plane bending vibrations of δC–H in an 1,4-substituted aromatic ring | - | - | 892 | 862 | - |
| Out-of-plane bending vibrations of δC–H in a mono-substituted aromatic ring | 699 | 696 | 710 | 709 | 697 |
| Property | PDPAC-1 | SWCNT/PDPAC-1 | PDPAC-2 | SWCNT/PDPAC-2 | ||
|---|---|---|---|---|---|---|
| 3 wt % | 10 wt % | 3 wt % | 10 wt % | |||
| * T5%, °C | 185/205 | 174/197 | 173/178 | 104/102 | 88/92 | 103/107 |
| ** T50%, °C | 523/663 | 544/834 | 536/>1000 | 517/396 | 473/774 | 518/789 |
| *** Residue, % | 20 | 44 | 51 | 35 | 45 | 46 |
| Property | PDPAC-1 | SWCNT/PDPAC-1 | PDPAC-2 | SWCNT/PDPAC-2 | ||
|---|---|---|---|---|---|---|
| 3 wt % | 10 wt % | 3 wt % | 10 wt % | |||
| * σ1, S/cm | 3.10 × 10−12 | 1.93 × 10−10 | 2.85 × 10−4 | 1.35 ×10−5 | 4.48 ×10−5 | 2.52 ×10−3 |
| ** σ2, S/cm | 1.12 ×10−7 | 1.87 × 10−6 | 3.32 × 10−4 | 2.17 ×10−5 | 8.07 ×10−5 | 5.15 ×10−3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkan, S.Z.; Karpacheva, G.P.; Kostev, A.I.; Bondarenko, G.N. Formation Features of Hybrid Nanocomposites Based on Polydiphenylamine-2-Carboxylic Acid and Single-Walled Carbon Nanotubes. Polymers 2019, 11, 1181. https://doi.org/10.3390/polym11071181
Ozkan SZ, Karpacheva GP, Kostev AI, Bondarenko GN. Formation Features of Hybrid Nanocomposites Based on Polydiphenylamine-2-Carboxylic Acid and Single-Walled Carbon Nanotubes. Polymers. 2019; 11(7):1181. https://doi.org/10.3390/polym11071181
Chicago/Turabian StyleOzkan, Sveta Zhiraslanovna, Galina Petrovna Karpacheva, Aleksandr Ivanovich Kostev, and Galina Nikolaevna Bondarenko. 2019. "Formation Features of Hybrid Nanocomposites Based on Polydiphenylamine-2-Carboxylic Acid and Single-Walled Carbon Nanotubes" Polymers 11, no. 7: 1181. https://doi.org/10.3390/polym11071181
APA StyleOzkan, S. Z., Karpacheva, G. P., Kostev, A. I., & Bondarenko, G. N. (2019). Formation Features of Hybrid Nanocomposites Based on Polydiphenylamine-2-Carboxylic Acid and Single-Walled Carbon Nanotubes. Polymers, 11(7), 1181. https://doi.org/10.3390/polym11071181