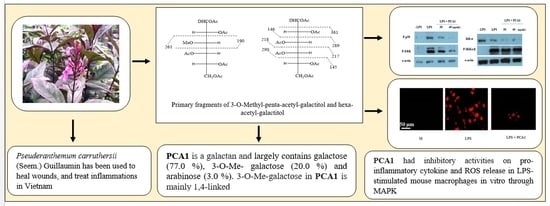
Neutral Polysaccharide from the Leaves of Pseuderanthemum carruthersii: Presence of 3-O-Methyl Galactose and Anti-Inflammatory Activity in LPS-Stimulated RAW 264.7 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Purification of Polysaccharides
2.3. Monosaccharide Composition
2.4. Linkage Analysis by Ethylation
2.5. NMR Spectroscopy
2.6. Determination of Molecular Weight of Polysaccharides
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Enzyme-Linked Immunosorbent Assay
2.10. Western Blotting Analysis
2.11. Measurement of Intracellular Reactive Oxygen Species (ROS)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Purification
3.2. Monosaccharide Composition and Molecular Weight of Polysaccharide
3.3. Linkage Analysis of PCA1
3.4. Structure Characterization by NMR Spectroscopy
3.5. Anti-Inflammatory Effect of PCA1 on LPS-Induced RAW 264.7 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pham, H.H. An Illustrated Flora of Vietnam, 2nd ed.; Tre Publishing House: Ho Chi Minh City, Vietnam, 2003; Volume 3, p. 67. [Google Scholar]
- Vo, T.N.; Nguyen, P.L.; Tuong, L.T.; Pratt, L.M.; Vo, P.N.; Nguyen, K.P.; Nguyen, N.S. Lignans and triterpenes from the root of Pseuderanthemum carruthersii var. atropurpureum. Chem. Pharm. Bull. 2012, 60, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Bac, V.H.; Huong, N.T.; Truong, L.V. Optimized extraction of polysaccaride from Pseuderanthemum caruthersii (Seem.) Guill. var. atropurpureum (Bull.) Fosb. J. Biol. 2018, 40, 162–167. [Google Scholar] [CrossRef]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.O.; Lee, S.H.; Park, D.K.; Choue, R.W. Effect of Dietary Pectin on the Production of Immunoglobulins and Cytokines by Mesenteric Lymph Node Lymphocytes in Mouse Colitis Induced with Dextran Sulfate Sodium. Biosci. Biotechnol. Biochem. 2003, 67, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vashishta, A.; Saraswat-Ohri, S.; Vetvickova, J. Immunological Effects of Yeast and Mushroom-Derived β-Glucans. J. Med. Food. 2008, 11, 615–622. [Google Scholar] [CrossRef]
- Santander, S.; Aoki, M.; Hernandez, J.; Pombo, M.; Moins-Teisserenc, H.; Mooney, N.; Fiorentino, S. Galactomannan from Caesalpinia spinosa induces phenotypic and functional maturation of human dendritic cells. Int. Immunopharmacol. 2011, 11, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.M.; Zhao, L.J.; Deng, J.; Xiong, S.H.; Tang, J.; Li, Y.M.; Xia, B.H.; Liao, D.F. Enzymatic Extraction, Purification, and Characterization of Polysaccharides from Penthorum chinense Pursh: Natural Antioxidant and Anti-Inflammatory. BioMed Res. Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Paiva, A.A.; Castro, A.J.; Nascimento, M.S.; Will, L.S.; Santos, N.D.; Araújo, R.M.; Xavier, C.A.; Rocha, F.A.; Leite, E.L. Antioxidant and anti inflammatory effect of polysaccharides from Lobophora variegata on zymosan-induced arthritis in rats. Int. Immunopharmacol. 2001, 11, 1241–1250. [Google Scholar] [CrossRef]
- Xie, G.; Schepetkin, I.A.; Siemsen, D.W.; Kirpotina, L.N.; Wiley, J.A.; Quinn, M.T. Fractionation and Characterization of Biologically-active Polysaccharides from Artemisia tripartita. Phytochemistry 2008, 69, 1359–1371. [Google Scholar] [CrossRef]
- Hokputsa, S.; Harding, S.E.; Inngjerdingen, K.; Jumel, K.; Michaelsen, T.E.; Heinze, T.; Koschella, A.; Paulsen, B.S. Bioactive polysaccharides from the stems of the Thai medicinal plant Acanthus ebracteatus: Their chemical and physical features. Carbohydr. Res. 2004, 339, 753–762. [Google Scholar] [CrossRef]
- Capek, P.; Hříbalová, V. Water-soluble polysaccharides from Salvia officinalis L. possessing immunomodulatory activity. Phytochemistry 2004, 65, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.L.; Smiderle, F.R.; Agostini, F.; Pereira, E.M.; Bonatti-Chaves, M.; Wisbeck, E.; Ruthes, A.C.; Sassaki, G.L.; Cipriani, T.R.; Furlan, S.A.; et al. Exopolysaccharide produced by Pleurotus sajor-caju: Its chemical structure and anti-inflammatory activity. Int. J. Biol. Macromol. 2015, 75, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Wold, C.W.; Kjeldsen, C.; Corthay, A.; Rise, F.; Christensen, B.E.; Duus, J.Ø.; Inngjerdingen, K.T. Structural characterization of bioactive heteropolysaccharides from the medicinal fungus Inonotus obliquus (Chaga). Carbohydr. Polym. 2018, 185, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Nagar, S.; Hensel, A.; Mischnick, P.; Kumar, V. A unique polysaccharide containing 3- O -methylarabinose and 3-O-methylgalactose from Tinospora sinensis. Carbohydr. Polym. 2018, 193, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Koschella, A.; Inngjerdingen, K.; Paulsen, B.S.; Morris, G.A.; Harding, S.E.; Heinze, T. Unconventional Methyl Galactan Synthesized via the Thexyldimethylsilyl Intermediate: Preparation, Characterization, and Properties. Macromol. Biosci. 2008, 8, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Boil. Chem. 1951, 193, 265–275. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Barsett, H.; Paulsen, B.S. Separation, isolation and characterization of acidic polysaccharides from the inner bark of Ulmus glabra Hudson. Carbohydr. Polym. 1991, 17, 137–144. [Google Scholar] [CrossRef]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 2012, 7, 1590–1607. [Google Scholar] [CrossRef]
- Barsett, H.; Paulsen, B.S.; Habte, Y. Further characterization of polysaccharides in seeds from Ulmus glabra Huds. Carbohydr. Polym. 1992, 18, 125–130. [Google Scholar] [CrossRef]
- Sims, I.M.; Bacic, A. Extracellular polysaccharides from suspension cultures of Nicotiana plumbaginifolia. Phytochemistry 1995, 38, 1397–1405. [Google Scholar] [CrossRef]
- Trinh, T.C.; Giang, H.D.; Tran Tr, D.; Nguyen, Q.H.; Nguyen, P.; Hoang, T.H. Wedelolactone from Vietnamese Eclipta prostrata (L) L. protected Zymosan-induced shock in Mice. Iran. J. Pharm. Res. 2018, 17, 653–660. [Google Scholar]
- Yang, C.S.; Ko, S.R.; Cho, B.G.; Shin, D.M.; Yuk, J.M.; Li, S.; Kim, J.M.; Evans, R.M.; Jung, J.S.; Song, D.K.; et al. The ginsenoside metabolite compound K, a novel agonist of glucocorticoid receptor, induces tolerance to endotoxin-induced lethal shock. J. Cell Mol. Med. 2018, 12, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Barsett, H.; Paulsen, B.S. Separation of acidic polysaccharides from Ulmus glabra Huds on Mono PTM. J. Chromatogr. 1985, 329, 315–320. [Google Scholar] [CrossRef]
- He, P.; Zhang, A.; Zhou, S.; Zhang, F.; Linhardt, R.J.; Sun, P. Structural elucidation of polysaccharide containing 3-O-methyl galactose from fruiting bodies of Pleurotus citrinopileatus. Carbohydr. Res. 2016, 434, 72–76. [Google Scholar] [CrossRef]
- Brito, D.R.; Carbonero, E.R.; Viana, S.R.; Silva, E.V.; Ruthes, A.C.; Lião, L.M.; Iacomini, M. Partially methylated galactans containing different proportions of 3-O-methyl galactose from Pleurotus citrinopileatus. Carbohydr. Res. 2018, 458, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, I.D.; Caillot, A.R.; Palhares, L.C.; Filho, A.P.; Chavante, S.F.; Sassaki, G.L. Structural characterization of polysaccharides from Cabernet Franc, Cabernet Sauvignon and Sauvignon Blanc wines: Anti-inflammatory activity in LPS stimulated RAW 264.7 cells. Carbohydr. Polym. 2018, 186, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Córdova, M.M.; Martins, D.F.; Silva, M.D.; Baggio, C.H.; Carbonero, E.R.; Ruthes, A.C.; Iacomini, M.; Santos, A.R. Polysaccharide glucomannan isolated from Heterodermia obscurata attenuates acute and chronic pain in mice. Carbohydr. Polym. 2013, 92, 2058–2064. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Tian, X.H.; Wang, Y.; Wang, D.D.; Li, W.; Chen, L.; Pan, W.J.; Mehmood, S.; Chen, Y. Immunomodulating activity of the polysaccharide TLH-3 from Tricholoma lobayense in RAW264.7 macrophages. Int. J. Biol. Macromol. 2017, 107, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.A.; Dias, E.G.; Brito, T.V.; Prudêncio, R.S.; Silva, R.O.; Ribeiro, R.A.; Souza, M.H.; De Paula, R.C.; Feitosa, J.P.; Chaves, L.S.; et al. Polysaccharide isolated from Agardhiella ramosissima: Chemical structure and anti-inflammation activity. Carbohydr. Polym. 2014, 99, 59–67. [Google Scholar] [CrossRef] [PubMed]











| Sugar Composition, Mw | PCA1 | PCA2 |
|---|---|---|
| Mw (Da) | 1.6826 × 105 | - |
| Monosaccharide composition * | ||
| Arabinose | 3.0 | 19.5 |
| Rhamnose | 3.7 | |
| Fucose | 1.8 | |
| Xylose | 2.3 | |
| Galactose | 77.0 | 50.4 |
| Glucuronic acid | 2.6 | |
| Galacturonic acid | 16.1 | |
| 3-O-Methyl galactose | 20.0 | 3.5 |
| Identity, Linkage Type, (Ratio) | Molar Masses of Primary Fragments of the Ethylated Alditol Acetates |
|---|---|
| 1,4 linked 3-O-methylgalactose (3,9) | 59, 132, 176, 247 |
| 1,4 linked galactose (1) | 59, 132, 190, 261 |
| Backbone | Chemical Shifts (Top: 13C, Bottom: 1H) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 6 | OCH3 | |
| →4)β-d-Gal(1→1 | 105.17 4.54 | 72.92 3.64 | 74.22 3.72 | 78.63 4.10 | 75.42 3.67 | 61.54 3.66 | 3.80 | |
| →4)α-d-Gal(1→1 | 101.30 4.98 | 69.82 3.85 | 69.69 3.91 | 79.59 4.07 | 72.36 4.32 | 60.93 3.75 | 3.83 | |
| β-1→4-Galp2 | 105.3 | 71.6 3.7 | 74.0 3.8 | 78.6 4.1 | 75.5 3.7 | 61.8–62.2 4.5–4.6 | 3.9 | |
| 3-O-Me-Galp2 | 104.5 | 71.6 3.7 | 85.64 3.8 | 78.6 4.1 | 75.5 3.7 | 61.8 4.5 | 62.2 4.6 | 58.64 3.5 |
| α-1→5- Araf2 | 108.5 | n. d. | n. d. | n. d. | 68.0 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bac, V.H.; Paulsen, B.S.; Truong, L.V.; Koschella, A.; Trinh, T.C.; Wold, C.W.; Yogarajah, S.; Heinze, T. Neutral Polysaccharide from the Leaves of Pseuderanthemum carruthersii: Presence of 3-O-Methyl Galactose and Anti-Inflammatory Activity in LPS-Stimulated RAW 264.7 Cells. Polymers 2019, 11, 1219. https://doi.org/10.3390/polym11071219
Bac VH, Paulsen BS, Truong LV, Koschella A, Trinh TC, Wold CW, Yogarajah S, Heinze T. Neutral Polysaccharide from the Leaves of Pseuderanthemum carruthersii: Presence of 3-O-Methyl Galactose and Anti-Inflammatory Activity in LPS-Stimulated RAW 264.7 Cells. Polymers. 2019; 11(7):1219. https://doi.org/10.3390/polym11071219
Chicago/Turabian StyleBac, Vo Hoai, Berit Smestad Paulsen, Le Van Truong, Andreas Koschella, Tat Cuong Trinh, Christian Winther Wold, Suthajini Yogarajah, and Thomas Heinze. 2019. "Neutral Polysaccharide from the Leaves of Pseuderanthemum carruthersii: Presence of 3-O-Methyl Galactose and Anti-Inflammatory Activity in LPS-Stimulated RAW 264.7 Cells" Polymers 11, no. 7: 1219. https://doi.org/10.3390/polym11071219
APA StyleBac, V. H., Paulsen, B. S., Truong, L. V., Koschella, A., Trinh, T. C., Wold, C. W., Yogarajah, S., & Heinze, T. (2019). Neutral Polysaccharide from the Leaves of Pseuderanthemum carruthersii: Presence of 3-O-Methyl Galactose and Anti-Inflammatory Activity in LPS-Stimulated RAW 264.7 Cells. Polymers, 11(7), 1219. https://doi.org/10.3390/polym11071219