A Simple Preparation Route for Bio-Phenol MQ Silicone Resin via the Hydrosilylation Method and its Autonomic Antibacterial Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
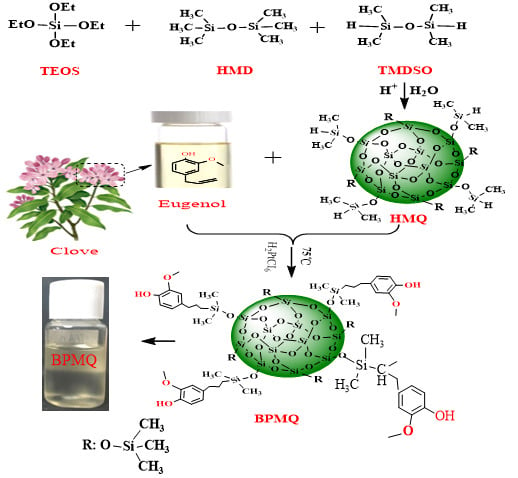
2.2. Synthesis of the HMQ
2.3. Synthesis of Bio-Phenol MQ Silicone Resin
2.4. Characterizations and Measurements
3. Results and Discussion
3.1. Structural Analysis
3.2. Viscosity Analysis
3.3. Thermal Property
3.4. Antibacterial Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, W.; Zeng, X.; Lai, X.; Li, H.; Pan, Z. Effect and mechanism of ureido-modified MQ silicone resin and platinum on tracking and erosion resistance of silicone rubber. Polym. Test. 2018, 70, 162–169. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, J.; Liu, X.; Ji, H.; He, R.; Pimhataivoot, P.; Chen, X. Versatile cascade esterification route to MQ Resins. ACS Omega 2018, 3, 4054–4062. [Google Scholar] [CrossRef]
- Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Song, Y.; Cui, Y.; Khan, M.; Ji, J.; et al. Reduced graphene oxide embedded with MQ silicone resin nano-aggregates for silicone rubber composites with enhanced thermal conductivity and mechanical performance. Polymers 2018, 10, 1254. [Google Scholar] [CrossRef]
- Ji, J.; Ge, X.; Pang, X.; Liang, W.; Wen, S.; Chen, X.; Yin, G.; Wen, M.; Ge, J. Preparation of self-Reinforced MQ cross-Linking agentandIts application in condensed room temperature vulcanization silicone rubber. Polym. Mater. Sci. Eng. 2019, 8, 1–9. [Google Scholar]
- Zhang, Y.; Huang, Y.; Liu, X.; Yu, Y. Studies on the silicone resins cured with polymethylsilazanes at ambient temperature. J. Appl. Polym. Sci. 2003, 89, 1702–1707. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, X.; Lai, X.; Li, H.; Zhou, Q.; Huang, X. Suppression effect and mechanism of amine-containing MQ silicone resin on the tracking and erosion resistance of silicone rubber. ACS Omega 2017, 2, 5111–5121. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Ji, J.; Ge, X.; Pang, X.; Liu, R.; Wen, S.; Sun, J.; Liang, W.; Ge, J.; Chen, X. Synthesis and characterization of room temperature vulcanized silicone rubber using methoxyl-capped MQ silicone resin as self-reinforced cross-linker. Polymers 2019, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.B.; Durfee, L.D.; Ekeland, R.A.; McVie, J.; Schalau, G.K. Recent advances in silicone pressure-sensitive adhesives. J. Adhes. Sci. Technol. 2007, 21, 605–623. [Google Scholar] [CrossRef]
- Mashak, A.; Rahimi, A. Silicone polymers in controlled drug delivery systems: A review. Iran. Polym. J. 2009, 18, 279–295. [Google Scholar]
- Shit, S.C.; Shah, P. A review on silicone rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Pang, X.; Ge, X.; Liang, W.; Chen, X.; Ge, J. Facile route for bio-phenol siloxane synthesis via heterogeneous catalytic method and its autonomic antibacterial property. Polymers 2018, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis of eugenol-based polybenzoxazine-POSS nanocomposites for low dielectric applications. Polym. Compos. 2015, 36, 1973–1982. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Eugenol and carvacrol migration from PHBV films and antibacterial action in different food matrices. Food Chem. 2019, 277, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Manrique, Y.; Gibis, M.; Schmidt, H.; Weiss, J. Antimicrobial efficacy of sequentially applied eugenol against food spoilage micro-organisms. J. Appl. Microbiol. 2016, 121, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, H.; Wimalasena, S.; De Silva, B.C.J.; Hossain, S.; Heo, G.-J. Antibacterial activity of clove essential oil and eugenol against fish pathogenic bacteria isolated from cultured olive flounder (Paralichthys olivaceus). Slov. Vet. Res. 2019, 56, 31–38. [Google Scholar]
- Dai, J.; Yang, S.; Teng, N.; Liu, Y.; Liu, X.; Zhu, J.; Zhao, J. Synthesis of eugenol-based silicon-containing benzoxazines and their applications as bio-based organic coatings. Coatings 2018, 8, 88. [Google Scholar] [CrossRef]
- Abitha, J. Role of antioxidants, anti-carcinogens and anti-mutagens. DJ J. Eng. Chem. Fuel 2016, 1, 8–16. [Google Scholar] [CrossRef]
- Ng, C.Y.; Yen, H.; Hsiao, H.Y.; Su, S.C. Phytochemicals in skin cancer prevention and treatment: An updated review. Int. J. Mol. Sci. 2018, 19, 941. [Google Scholar] [CrossRef]
- Xu, J.G.; Liu, T.; Hu, Q.P.; Cao, X.M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Xu, X.; Wu, C.; Zhang, B.; Dong, H. Preparation, structure characterization, and thermal performance of phenyl-modified MQ silicone resins. J. Appl. Polym. Sci. 2013, 128, 4189–4200. [Google Scholar] [CrossRef]
- Hochgatterer, N.S.; Schweiger, M.R.; Koller, S.; Raimann, P.R.; Wöhrle, T.; Wurm, C.; Winter, M. Silicon/graphite composite electrodes for high-capacity anodes: Influence of binder chemistry on cycling stability. Electrochem. Solid-State Lett. 2008, 11, A76–A80. [Google Scholar] [CrossRef]
- Haidary, S.M.; Mohammed, A.B.; Córcoles, E.P.; Ali, N.K.; Ahmadd, M.R. Effect of coatings and surface modification on porous silicon nanoparticles for delivery of the anticancer drug tamoxifen. Microelectron. Eng. 2016, 2016. 161, 1–6. [Google Scholar] [CrossRef]
- Maegawa, T.; Irie, Y.; Imoto, H.; Fueno, H.; Tanaka, K.; Naka, K. Para-bisvinylhexaisobutyl-substituted T8 caged monomer: synthesis and hydrosilylation polymerization. Polym. Chem. 2015, 6, 7500–7504. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, A.S.; Lee, H.S.; Jeon, H.Y.; Baek, K.Y.; Choi, D.H.; Hwang, S.S. Synthesis and chara- cterization of UV-curable ladder-like polysilsesquioxane. J. Polym. Sci. A Polym. Chem. 2011, 49, 5012–5018. [Google Scholar] [CrossRef]
- Li, R.; Zhang, B.; Sun, Y.; Liu, B.; Wang, G. Synthesis of vinylphenyl oligomeric silsesquioxane based on MQ silicone resin. Asian J. Chem. 2013, 25, 2541–2546. [Google Scholar] [CrossRef]
- Garg, A.; Gupta, B.; Prakash, R.; Singh, S. Preparation and characterization of hydroxypropyl-β-cyclodextrin inclusion complex of eugenol: differential pulse voltammetry and 1H-NMR. Chem. Pharm. Bull. 2010, 58, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, M.; Cateni, F.; Procida, G. Improvement of chemical and physical properties and antioxidant evaluation of eugenol–PEG adduct. Nat. Prod. Commun. 2017, 12, 413–416. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Sun, W.; Chen, W.; Dong, J.; Wen, J.; Zhang, J.; Li, Z.; Zheng, L.; Chen, C.; et al. Discovering partially charged single-atom Pt for enhanced anti-markovnikov alkene hydros-ilylation. J. Am. Chem. Soc. 2018, 140, 7407–7410. [Google Scholar] [CrossRef]
- Antosik, A.K.; Czech, Z. Pressure-sensitive adhesives (PSA) based on silicone. Adv. Mater. Interfaces 2016, 7, 249–274. [Google Scholar]
- Hartmann-Thompson, C.; Keeley, D.L.; Dvornic, P.R.; Keinath, S.E.; McCrea, K.R. Hydrogen-bond acidic polyhedral oligosilsesquioxane filled polymer coatings for surface acoustic wave sensors. J. Appl. Polym. Sci. 2007, 104, 3171–3182. [Google Scholar] [CrossRef]
- Dalsin, S.J.; Hillmyer, M.A.; Bates, F.S. Molecular weight dependence of zero-shear viscosity in atactic polypropylene bottlebrush polymers. ACS Macro Lett. 2014, 3, 423–427. [Google Scholar] [CrossRef]
- Mu, Q.; Peng, D.; Ju, W.; Zhang, F.; Wang, C. Synthesis and effects of MDT silicone resin on PMPS-based ablative composites. J. Appl. Polym. Sci. 2015, 132, 41571. [Google Scholar] [CrossRef]
- Almaroof, A.; Niazi, S.A.; Rojo, L.; Mannocci, F.; De, S. Influence of a polymerizable eugenol derivative on the antibacterial activity and wettability of a resin composite for intracanal post cementation and core build-up restoration. Dent. Mater. 2016, 32, 929–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidayah, A.N.; Wasito, E.B.; Debora, K.; Basori, A.; Isnaeni, I.; Utomo, B. Correlation between the bacteriostatic and bactericide effect with antibiofilm and anticolony spreading from javanese citronella oil on methicillin-resistant staphylococcus aureus (MRSA). Folia Medica Indonesiana 2019, 55, 1–9. [Google Scholar]
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-modified polysiloxanes as effective anticorrosion additives for epoxy resin coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef] [Green Version]






| Entry | Mw (dal.mol−1) | Mn (dal.mol−1) | Mw/Mn | Dynamic Viscosity (mpa.s) |
|---|---|---|---|---|
| HMQ | 2000 | 1400 | 1.43 | 440 |
| BPMQ | 2600 | 1800 | 1.44 | 465 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Ge, X.; Liang, W.; Liang, R.; Pang, X.; Liu, R.; Wen, S.; Sun, J.; Chen, X.; Ge, J. A Simple Preparation Route for Bio-Phenol MQ Silicone Resin via the Hydrosilylation Method and its Autonomic Antibacterial Property. Polymers 2019, 11, 1389. https://doi.org/10.3390/polym11091389
Ji J, Ge X, Liang W, Liang R, Pang X, Liu R, Wen S, Sun J, Chen X, Ge J. A Simple Preparation Route for Bio-Phenol MQ Silicone Resin via the Hydrosilylation Method and its Autonomic Antibacterial Property. Polymers. 2019; 11(9):1389. https://doi.org/10.3390/polym11091389
Chicago/Turabian StyleJi, Jianye, Xin Ge, Weijie Liang, Ruiyuan Liang, Xiaoyan Pang, Ruoling Liu, Shuyi Wen, Jiaqi Sun, Xunjun Chen, and Jianfang Ge. 2019. "A Simple Preparation Route for Bio-Phenol MQ Silicone Resin via the Hydrosilylation Method and its Autonomic Antibacterial Property" Polymers 11, no. 9: 1389. https://doi.org/10.3390/polym11091389
APA StyleJi, J., Ge, X., Liang, W., Liang, R., Pang, X., Liu, R., Wen, S., Sun, J., Chen, X., & Ge, J. (2019). A Simple Preparation Route for Bio-Phenol MQ Silicone Resin via the Hydrosilylation Method and its Autonomic Antibacterial Property. Polymers, 11(9), 1389. https://doi.org/10.3390/polym11091389