Enhanced Solubility, Stability, and Herbicidal Activity of the Herbicide Diuron by Complex Formation with β-Cyclodextrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Method of the Inclusion Complex
2.3. Preparation of the Physical Mixture
2.4. Characterization Method
2.4.1. FTIR Analysis
2.4.2. XRD Analysis
2.4.3. SEM Study
2.4.4. Phase Solubility Study
2.4.5. Determination of the Inclusion Ratio
2.4.6. TGA Study
2.4.7. DSC Study
2.4.8. 1H NMR Study
2.4.9. Detection of Biological Activity
3. Results
3.1. FTIR Analysis
3.2. XRD Analysis
3.3. SEM Analysis
3.4. Phase Solubility Study
3.5. Inclusion Ratio Study
3.6. TGA Study
3.7. DSC Results
3.8. 1H NMR Analysis
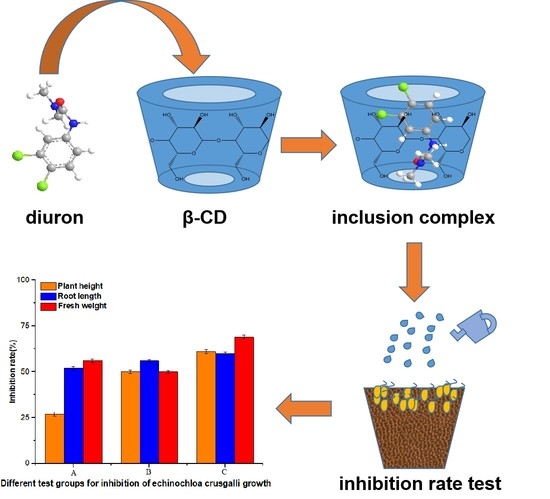
3.9. Results of the Biological Activity Assay
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Tixier, C.; Bogaerts, P.; Sancelme, M.; Bonnemoy, F.; Twagilimana, L.; Cuer, A.; Bohatier, J.; Veschambre, H. Fungal biodegradation of a phenylurea herbicide, diuron: Structure and toxicity of metabolites. Pest Manage. Sci. 2015, 56, 455–462. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Y.; Li, N.; Qian, Y.; Wang, Z.; Liu, Y. A national-scale cumulative exposure assessment of organophosphorus pesticides through dietary vegetable consumption in China. Food Control 2019, 104, 34–41. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Y.X.; Yi, K.H.; Li, M.Q.; Li, J.Z.; Ye, F. Quantitative Structure Activity Relationship Studies and Molecular Dynamics Simulations of 2-(Aryloxyacetyl)cyclohexane-1,3-Dones Derivatives as 4-Hydroxyphenylpyruvate Dioxygenase Inhibitors. Front. Chem. 2019, 7, 556. [Google Scholar] [CrossRef]
- Gao, S.; Bie, C.; Ji, Q.Y.; Ling, H.Y.; Li, C.Y.; Fu, Y.; Zhao, L.X.; Ye, F. Preparation and characterization of cyanazine–hydroxypropyl-beta-cyclodextrin inclusion complex. RSC Adv. 2019, 9, 26109–26115. [Google Scholar] [CrossRef]
- TONELLI, Alan, E. Molecular Processing of Polymers with Cyclodextrins. Adv. Polym. Sci. 2009, 222, 115–166. [Google Scholar]
- Martina, K.; Cravotto, G. Cyclodextrin as a Food Additive in Food Processing. In Functional Polymers in Food Science: From Technology to Biology, 1st ed.; Cirillo, G., Spizzirri, U.G., Iemma, F., Eds.; Scrivener Publishing: Beverly, Italy, 2015; Volume 1, pp. 267–288. ISBN 9781118595183. [Google Scholar]
- Rubim, A.M.; Rubenick, J.B.; Maurer, M.; Laporta, L.V.; Bueno Rolim, C.M. Inclusion complex of amiodarone hydrochloride with cyclodextrins: Preparation, characterization and dissolution rate evaluation. Braz. J. Pharm. Sci. 2017, 53. [Google Scholar] [CrossRef]
- Lv, P.; Liu, M.; Liao, R.; Zhao, Y.; Liao, X.; Gao, C.; Yang, B. Host-guest inclusion system of rhein with polyamine-modified β-cyclodextrins: Characterization and cytotoxicity. Pharm. Dev. Technol. 2016, 22, 1–26. [Google Scholar] [CrossRef]
- Gao, S.; Bie, C.; Liu, Y.; Zhang, T.; Fu, Y.; Ye, F. Functional Supramolecular of Inclusion Complex of Herbicide Fluroxypyr with HPβCD. Polymers 2018, 10, 1294. [Google Scholar] [CrossRef]
- Moreira, A.M.D.; Bittencourt, V.C.E.; Costa, F.L.S.; de Lima, M.E.; Lopes, M.T.P.; Borges, W.S.; Martins, G.F.; Nascimento, C.S.; da Silva, J.G.; Denadai, A.M.L. Hydrophobic Nanoprecipitates of beta-Cyclodextrin/Avermectins Inclusion Compounds Reveal Insecticide Activity against Aedes aegypti Larvae and Low Toxicity against Fibroblasts. J. Agric. Food Chem. 2018, 66, 7275–7285. [Google Scholar] [CrossRef]
- Geng, Q.Q.; Xie, J.C.; Wang, X.; Cai, M.L.; Ma, H.; Ni, H.W. Preparation and Characterization of Butachlor/(2-Hydroxypropyl)-beta-cyclodextrin Inclusion Complex: Improve Soil Mobility and Herbicidal Activity and Decrease Fish Toxicity. J. Agric. Food Chem. 2018, 66, 12198–12205. [Google Scholar] [CrossRef]
- Ren, X.; Yue, S.; Hong, X.; Xie, M. Inclusion complexes of eucalyptus essential oil with β-cyclodextrin: Preparation, characterization and controlled release. J. Porous Mat. 2018, 25, 1577–1586. [Google Scholar] [CrossRef]
- Pires, F.Q.; Pinho, L.A.; Freire, D.O.; Silva, I.C.R.; Sa-Barreto, L.L.; Cardozo, L.; Gratieri, T.; Gelfuso, G.M.; Cunha, M. Thermal analysis used to guide the production of thymol and Lippia origanoides essential oil inclusion complexes with cyclodextrin. J. Therm. Anal. 2019, 137, 543–553. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Y.; Xie, X.; Chen, F.; Li, W. Molecular Dynamics Simulation and TDDFT Study of the Structures and Uv-vis Absorption Spectra of MCT-β-CD and its Inclusion Complexes. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2015, 149, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, K.; Wang, P.; Kang, J.X.; Gao, S.; Zhao, L.X.; Ye, F. Design, Synthesis, and Herbicidal Activity Evaluation of Novel Aryl-Naphthyl Methanone Derivatives. Front. Chem. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, S.Q.; Liu, Y.X.; Wang, J.Y.; Gao, S.; Zhao, L.X.; Ye, F. Design, synthesis, SAR and molecular docking of novel green niacin-triketone HPPD inhibitor. Ind. Crop. Prod. 2019, 137, 566–575. [Google Scholar] [CrossRef]
- Ye, F.; Zhai, Y.; Kang, T.; Wu, S.L.; Li, J.J.; Gao, S.; Zhao, L.X.; Fu, Y. Rational design, synthesis and structure-activity relationship of novel substituted oxazole isoxazole carboxamides as herbicide safener. Pest. Biochem. Physiol. 2019, 157, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, Y.Y.; Jiang, J.Y.; Ji, Q.Y.; Fu, Y.; Zhao, L.X.; Li, C.Y.; Ye, F. Protective Responses Induced by Chiral 3-Dichloroacetyl Oxazolidine Safeners in Maize (Zea mays L.) and the Detoxification Mechanism. Molecules 2019, 24, 3060. [Google Scholar] [CrossRef]
- Ye, F.; Ma, P.; Zhang, Y.Y.; Li, P.; Yang, F.; Fu, Y. Herbicidal Activity and Molecular Docking Study of Novel ACCase Inhibitors. Front. Plant Sci. 2018, 9, 1850. [Google Scholar] [CrossRef]
- Tutunchi, P.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Extraction of red beet extract with beta-cyclodextrin-enhanced ultrasound assisted extraction: A strategy for enhancing the extraction efficacy of bioactive compounds and their stability in food models. Food Chem. 2019, 297, 124994. [Google Scholar] [CrossRef]
- Mokhtar, M.S.; Suliman, F.O.; Elbashir, A.A. Atrazine and ametryne inclusion complexes with 2-hydroxypropyl-beta/gamma-cyclodextrin: Spectroscopic studies and molecular dynamics simulation. J. Mol. Struct. 2019, 1179, 161–170. [Google Scholar] [CrossRef]
- Geng, Q.; Li, T.; Wang, X.; Chu, W.; Cai, M.; Xie, J.; Ni, H. The mechanism of bensulfuron-methyl complexation with beta-cyclodextrin and 2-hydroxypropyl-beta-cyclodextrin and effect on soil adsorption and bio-activity. Sci. Rep. 2019, 9, 1882. [Google Scholar] [CrossRef] [PubMed]
- Mahpishanian, S.; Sereshti, H. One-step green synthesis of β-cyclodextrin/iron oxide-reduced graphene oxide nanocomposite with high supramolecular recognition capability: Application for vortex-assisted magnetic solid phase extraction of organochlorine pesticides residue from honey sam. J. Chromatogr. A 2017, 1485, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.P.; Balakrishnan, S.B.; Ganesan, V.; Munisamy, M.; Kuppu, S.V.; Narayanan, V.; Baskaralingam, V.; Jeyachandran, S.; Thambusamy, S. In-vitro dissolution and microbial inhibition studies on anticancer drug etoposide with beta-cyclodextrin. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 102, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Lu, Z.; Jin, Z. Characterization of an inclusion complex of ethyl benzoate with hydroxypropyl-β-cyclodextrin. Food Chem. 2014, 152, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, S.; Daniel, G.; Nicolás, Y.; Paul, J. Removal of Aromatic Chlorinated Pesticides from Aqueous Solution Using β-Cyclodextrin Polymers Decorated with Fe₃O₄ Nanoparticles. Polymers 2018, 10, 1038. [Google Scholar]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin-β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Osada, M.; Murata, I.; Kobata, K.; Kanamoto, I. Evaluation of Solubility Characteristics of a Hybrid Complex of Components of Soy. Acs Omega 2019, 4, 8632–8640. [Google Scholar] [CrossRef] [Green Version]
- Mona, S.; Mitali, P.; Devendra, S.; Ajay, S. Cyclodextrin Inclusion Complex of Racecadotril: Effect of Drug-β-Cyclodextrin Ratio and the Method of Complexation. Curr. Drug Discovery Technol. 2014, 11, 154–161. [Google Scholar]
- Pooresmaeil, M.; Namazi, H. Surface modification of graphene oxide with stimuli-responsive polymer brush containing beta-cyclodextrin as a pendant group: Preparation, characterization, and evaluation as controlled drug delivery agent. Colloid Surf. B-Biointerfaces 2018, 172, 17–25. [Google Scholar] [CrossRef]
- Cui, H.Y.; Siva, S.; Lin, L. Ultrasound processed cuminaldehyde/2-hydroxypropyl-beta-cyclodextrin inclusion complex: Preparation, characterization and antibacterial activity. Ultrason. Sonochem. 2019, 56, 84–93. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Qi, L.; Du, Q.; Xia, Y. Kinetic, Isotherm and Thermodynamic Studies for Removal of Methylene Blue Using β-Cyclodextrin/Activated Carbon Aerogels. J. Polym. Environ. 2018, 26, 1–9. [Google Scholar] [CrossRef]
- Zhang, N.; Shen, J.; Pasquinelli, M.A.; Hinks, D.; Tonelli, A.E. Formation and characterization of an inclusion complex of triphenyl phosphate and β-cyclodextrin and its use as a flame retardant for polyethylene terephthalate. Polym. Degrad. Stabil. 2015, 120, 244–250. [Google Scholar] [CrossRef]
- Whang, H.S.; Tonelli, A. Release characteristics of the non-toxic insect repellant 2-undecanone from its crystalline inclusion compound with α-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 127–134. [Google Scholar] [CrossRef]
- Gurarslan, A.; Joijode, A.S.; Tonelli, A.E. Polymers coalesced from their cyclodextrin inclusion complexes: What can they tell us about the morphology of melt-crystallized polymers? J. Polym. Sci. Pt. B-Polym. Phys. 2012, 50, 813–823. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.Y.; Jiang, J.Y.; Ji, Q.Y.; Fu, Y.; Zhao, L.X.; Li, C.Y.; Ye, F. Physicochemical properties and fungicidal activity of inclusion complexes of fungicide chlorothalonil with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Liq. 2019, 293, 111513. [Google Scholar] [CrossRef]
- Abdala, A.A.; Tonelli, A.E.; Khan, S.A. Modulation of hydrophobic interactions in associative polymers using inclusion compounds and surfactants. Macromolecules 2003, 36, 7833–7841. [Google Scholar] [CrossRef]
- Narayanan, G.; Gupta, B.S.; Tonelli, A.E. Estimation of the poly (epsilon-caprolactone) PCL and alpha-cyclodextrin alpha-CD stoichiometric ratios in their inclusion complexes ICs, and evaluation of porosity and fiber alignment in PCL nanofibers containing these ICs. Data in brief 2015, 5, 1048–1055. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Pereira, A.E.S.; Ribeiro, L.N.; Fernandes, F.O.; Gonçalves, K.C.; Polanczyk, R.A.; Pasquotostigliani, T.; Lima, R. Carvacrol and linalool co-loaded in β-cyclodextrin-grafted chitosan nanoparticles as sustainable biopesticide aiming pest control. Sci. Rep. 2018, 8, 7623. [Google Scholar] [CrossRef]












| Compounds | Chemical Shift (δ) | |||||
|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-6 | H-5 | |
| Diuron | 8.575 | 7.865 | 7.475 | 3.367 | 2.928 | 2.518 |
| Inclusion complex of β-CD and diuron | 8.551 | 7.845 | 7.461 | 3.335 | 2.921 | 2.507 |
| Δδ | 0.024 | 0.020 | 0.014 | 0.032 | 0.007 | 0.011 |
| β-CD | 5.750 | 3.347 | 3.653 | 3.300 | 3.629 | 3.603 |
| Inclusion complex of β-CD and diuron | 5.740 | 3.338 | 3.629 | 3.293 | 3.623 | 3.571 |
| Δδ | 0.010 | 0.009 | 0.024 | 0.007 | 0.006 | 0.032 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Jiang, J.-Y.; Liu, Y.-Y.; Fu, Y.; Zhao, L.-X.; Li, C.-Y.; Ye, F. Enhanced Solubility, Stability, and Herbicidal Activity of the Herbicide Diuron by Complex Formation with β-Cyclodextrin. Polymers 2019, 11, 1396. https://doi.org/10.3390/polym11091396
Gao S, Jiang J-Y, Liu Y-Y, Fu Y, Zhao L-X, Li C-Y, Ye F. Enhanced Solubility, Stability, and Herbicidal Activity of the Herbicide Diuron by Complex Formation with β-Cyclodextrin. Polymers. 2019; 11(9):1396. https://doi.org/10.3390/polym11091396
Chicago/Turabian StyleGao, Shuang, Jing-Yu Jiang, Yan-Yan Liu, Ying Fu, Li-Xia Zhao, Chun-Yan Li, and Fei Ye. 2019. "Enhanced Solubility, Stability, and Herbicidal Activity of the Herbicide Diuron by Complex Formation with β-Cyclodextrin" Polymers 11, no. 9: 1396. https://doi.org/10.3390/polym11091396
APA StyleGao, S., Jiang, J. -Y., Liu, Y. -Y., Fu, Y., Zhao, L. -X., Li, C. -Y., & Ye, F. (2019). Enhanced Solubility, Stability, and Herbicidal Activity of the Herbicide Diuron by Complex Formation with β-Cyclodextrin. Polymers, 11(9), 1396. https://doi.org/10.3390/polym11091396