Preparation of Phase Change Microcapsules with the Enhanced Photothermal Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
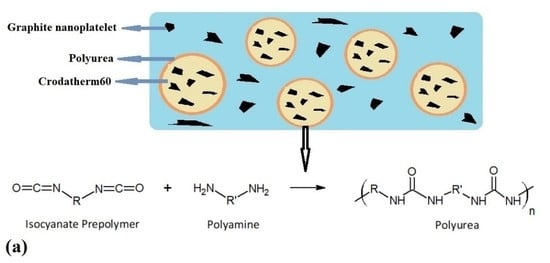
2.2. Fabrication of Cr60@Polyurea Microcapsules
2.3. Fabrication of Cr60@Polyurea-GNP Microcapsules
2.4. Photothermal Slurry Preparation
2.5. Characterization Methods
2.6. Photothermal Performance Analysis
3. Results and Discussion
3.1. Chemical Characterization of Microcapsules
3.2. Surface Morphology of Microcapsules
3.3. Thermal Characteristics of Microcapsules
3.4. Thermal Stability of Microcapsules
3.5. Rheology Behaviour (Viscosity) of the MEPCM Slurry
3.6. Optical Characterization of the MEPCM Slurry (UV-Vis-NIR)
3.7. Photothermal Measurement
3.8. Receiver Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Zheng, C.; Mesgari, S.; Hewkuruppu, Y.L.; Hjerrild, N.; Crisostomo, F.; Rosengarten, G.; Scott, J.A.; Taylor, R.A. Experimental and numerical investigation of volumetric versus surface solar absorbers for a concentrated solar thermal collector. Sol. Energy 2016, 136, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Gorji, T.B.; Ranjbar, A. A review on optical properties and application of nanofluids in direct absorption solar collectors (DASCs). Renew. Sustain. Energy Rev. 2017, 72, 10–32. [Google Scholar] [CrossRef]
- Suman, S.; Khan, M.K.; Pathak, M. Performance enhancement of solar collectors—A review. Renew. Sustain. Energy Rev. 2015, 49, 192–210. [Google Scholar] [CrossRef]
- Ge, T.; Wang, R.; Xu, Z.; Pan, Q.; Du, S.; Chen, X.; Ma, T.; Wu, X.; Sun, X.; Chen, J. Solar heating and cooling: Present and future development. Renew. Energy 2018, 126, 1126–1140. [Google Scholar] [CrossRef]
- Hussein, A.K.; Walunj, A.; Kolsi, L. Applications of nanotechnology to enhance the performance of the direct absorption solar collectors. J. Therm. Eng. 2016, 2, 529–540. [Google Scholar] [CrossRef]
- Sabiha, M.A.; Saidur, R.; Mekhilef, S.; Mahian, O. Progress and latest developments of evacuated tube solar collectors. Renew. Sustain. Energy Rev. 2015, 51, 1038–1054. [Google Scholar] [CrossRef]
- Qin, C.; Kang, K.; Lee, I.; Lee, B.J. Optimization of a direct absorption solar collector with blended plasmonic nanofluids. Sol. Energy 2017, 150, 512–520. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Kyritsis, D.C.; Abu-Nada, E. Impact of nanofluids, radiation spectrum, and hydrodynamics on the performance of direct absorption solar collectors. Energy Convers. Manag. 2018, 156, 706–722. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.N.; Mehrali, M.; Zubir, M.N.B.M.; Metselaar, H.S.C. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 15. [Google Scholar] [CrossRef]
- Otanicar, T.P.; Phelan, P.E.; Golden, J.S. Optical properties of liquids for direct absorption solar thermal energy systems. Sol. Energy 2009, 83, 969–977. [Google Scholar] [CrossRef]
- Ladjevardi, S.; Asnaghi, A.; Izadkhast, P.; Kashani, A. Applicability of graphite nanofluids in direct solar energy absorption. Sol. Energy 2013, 94, 327–334. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Zeng, S.; Zheng, Z. Experimental investigation on photothermal properties of nanofluids for direct absorption solar thermal energy systems. Energy Convers. Manag. 2013, 73, 150–157. [Google Scholar] [CrossRef]
- Mehrali, M.; Ghatkesar, M.K.; Pecnik, R. Full-spectrum volumetric solar thermal conversion via graphene/silver hybrid plasmonic nanofluids. Appl. Energy 2018, 224, 103–115. [Google Scholar] [CrossRef]
- Tyagi, H.; Phelan, P.; Prasher, R. Predicted efficiency of a low-temperature nanofluid-based direct absorption solar collector. J. Sol. Energy Eng. 2009, 131, 041004. [Google Scholar] [CrossRef]
- Zhao, W.; France, D.M.; Yu, W.; Kim, T.; Singh, D. Phase change material with graphite foam for applications in high-temperature latent heat storage systems of concentrated solar power plants. Renew. Energy 2014, 69, 134–146. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Indra Mahlia, T.M.; Cornelis Metselaar, H.S.; Naghavi, M.S.; Sadeghinezhad, E.; Akhiani, A.R. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640. [Google Scholar] [CrossRef]
- Verma, V.; Kundan, L. Thermal performance evaluation of a direct absorption flat plate solar collector (DASC) using Al2O3-H2O based nanofluids. IOSR J. Mech. Civ. Eng. 2013, 6, 29–35. [Google Scholar] [CrossRef]
- Akhiani, A.R.; Mehrali, M.; Tahan Latibari, S.; Mehrali, M.; Mahlia, T.M.I.; Sadeghinezhad, E.; Metselaar, H.S.C. One-Step Preparation of Form-Stable Phase Change Material through Self-Assembly of Fatty Acid and Graphene. J. Phys. Chem. C 2015, 119, 22787–22796. [Google Scholar] [CrossRef]
- Tahan Latibari, S.; Mehrali, M.; Mehrali, M.; Afifi, A.B.M.; Mahlia, T.M.I.; Akhiani, A.R.; Metselaar, H.S.C. Facile synthesis and thermal performances of stearic acid/titania core/shell nanocapsules by sol–gel method. Energy 2015, 85, 635–644. [Google Scholar] [CrossRef]
- Tahan Latibari, S.; Mehrali, M.; Mehrali, M.; Indra Mahlia, T.M.; Cornelis Metselaar, H.S. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Innovative design of microencapsulated phase change materials for thermal energy storage and versatile applications: A review. Sustain. Energy Fuels 2019, 3, 1091–1149. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Characterization of polymethyl methacrylate/polyethylene glycol/aluminum nitride composite as form-stable phase change material prepared by in situ polymerization method. Thermochim. Acta 2011, 524, 128–134. [Google Scholar]
- Yang, R.; Xu, H.; Zhang, Y. Preparation, physical property and thermal physical property of phase change microcapsule slurry and phase change emulsion. Sol. Energy Mater. Sol. Cells 2003, 80, 405–416. [Google Scholar] [CrossRef]
- Xiao, D.; Qu, Y.; Hu, S.; Han, H.; Li, Y.; Zhai, J.; Jiang, Y.; Yang, H. Study on the phase change thermal storage performance of palmitic acid/carbon nanotubes composites. Compos. Part A Appl. Sci. Manuf. 2015, 77, 50–55. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Fang, X.; Zhang, Z. Graphite nanoparticles-dispersed paraffin/water emulsion with enhanced thermal-physical property and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2016, 147, 101–107. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, H.; Liu, J.; Fang, X.; Zhang, Z. Novel slurry containing graphene oxide-grafted microencapsulated phase change material with enhanced thermo-physical properties and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2015, 143, 29–37. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.; Chan, C. Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: A review to recent developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef]
- Calle, M.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Non-isocyanate route to biobased polyurethanes and polyureas via AB-type self-polycondensation. Eur. Polym. J. 2016, 84, 837–848. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Tahan Latibari, S.; Mehrali, M.; Togun, H.; Zubir, M.N.M.; Kazi, S.N.; Metselaar, H.S.C. Preparation, characterization, viscosity, and thermal conductivity of nitrogen-doped graphene aqueous nanofluids. J. Mater. Sci. 2014, 49, 7156–7171. [Google Scholar] [CrossRef]
- Bejan, A. Convection Heat Transfer, 2nd ed.; Wiley: New York, NY, USA, 1993. [Google Scholar]











| Sample Code | Tm,peak (°C) | ΔHm (J/g) | Tc,peak (°C) | ΔHc (J/g) | Encapsulation Ratio (%) | Encapsulation Efficiency (%) | Thermal Storage Capacity (%) |
|---|---|---|---|---|---|---|---|
| Cr60 | 61.9 | 218.9 | 56.8 | 206.0 | - | - | - |
| Cr60@Polyurea | 64.6 | 61.6 | 25.2 | 24.9 | 28.1 | 20.4 | 72.3 |
| [email protected]%GNP | 64.3 | 95.5 | 53.9 | 87.5 | 43.6 | 43.1 | 98.7 |
| Sample Code | ∆mtot (%) | ∆mStep1 (%) | ∆mStep2 (%) | Tp1 (°C) | T0 (°C) | Tp2 (°C) |
|---|---|---|---|---|---|---|
| [email protected] wt %GNP | −96 | −21 | −75 | 155 | 280 | 315 |
| Cr60@polyurea | −92 | −28 | −59 | 153 | 252 | 312 |
| Crodatherm60 | −100 | - | −95 | - | - | 304 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahan Latibari, S.; Eversdijk, J.; Cuypers, R.; Drosou, V.; Shahi, M. Preparation of Phase Change Microcapsules with the Enhanced Photothermal Performance. Polymers 2019, 11, 1507. https://doi.org/10.3390/polym11091507
Tahan Latibari S, Eversdijk J, Cuypers R, Drosou V, Shahi M. Preparation of Phase Change Microcapsules with the Enhanced Photothermal Performance. Polymers. 2019; 11(9):1507. https://doi.org/10.3390/polym11091507
Chicago/Turabian StyleTahan Latibari, Sara, Jacco Eversdijk, Ruud Cuypers, Vassiliki Drosou, and Mina Shahi. 2019. "Preparation of Phase Change Microcapsules with the Enhanced Photothermal Performance" Polymers 11, no. 9: 1507. https://doi.org/10.3390/polym11091507
APA StyleTahan Latibari, S., Eversdijk, J., Cuypers, R., Drosou, V., & Shahi, M. (2019). Preparation of Phase Change Microcapsules with the Enhanced Photothermal Performance. Polymers, 11(9), 1507. https://doi.org/10.3390/polym11091507