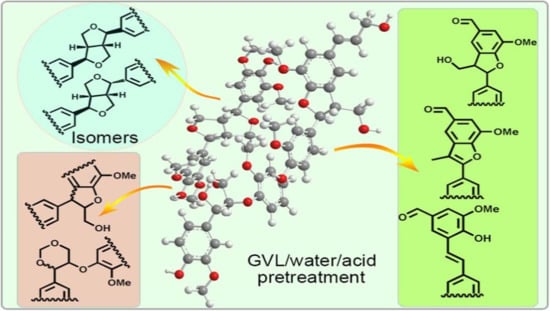
Revealing Structural Modifications of Lignin in Acidic γ-Valerolactone-H2O Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Acid Pretreatment of Lignin Models in GVL/ H2O (80/20) System
2.3. GC-MS Measurements
2.4. NMR Measurements
2.5. Synthesis of 1,3-Dioxane Compound 1
3. Results
3.1. Reactions of β-O-4 Lignin Model GG in GVL/ H2O (80/20) System
3.2. Reactions of β-5 Lignin Models in GVL/ H2O (80/20) System
3.3. Reactions of β-β Lignin Model in GVL/ H2O (80/20) System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Wen, J.L.; Yuan, T.Q.; Sun, S.L.; Xu, F.; Sun, R.C. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Sankar, M.; Beale, A.M.; He, Q.; Kiely, C.J.; Bruijnincx, P.C.; Weckhuysen, B.M. High performing and stable supported nano-alloys for the catalytic hydrogenation of levulinic acid to γ-valerolactone. Nat. Commun. 2015, 6, 6540. [Google Scholar] [CrossRef] [PubMed]
- Luterbacher, J.S.; Rand, J.M.; Alonso, D.M.; Han, J.; Youngquist, J.T.; Maravelias, C.T.; Pfleger, B.F.; Dumesic, J.A. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 2014, 343, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Sixta, H. Advanced biorefinery based on the fractionation of biomass in γ-valerolactone and water. ChemSusChem 2015, 8, 73–76. [Google Scholar] [CrossRef]
- Li, S.X.; Li, M.F.; Yu, P.; Fan, Y.M.; Shou, J.N.; Sun, R.C. Valorization of bamboo by γ-valerolactone/acid/water to produce digestible cellulose, degraded sugars and lignin. Bioresour. Technol. 2017, 230, 90–96. [Google Scholar] [CrossRef]
- Li, Y.J.; Li, H.Y.; Cao, X.F.; Sun, S.N.; Sun, R.C. Understanding the distribution and structural feature of eucalyptus lignin isolated by γ-valerolactone/water/acid system. ACS Sustain. Chem. Eng. 2018, 6, 12124–12131. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, W.; Wang, Z.; Ma, Y.; Jiang, L.; Wang, T. Efficient degradation of lignin in raw wood via pretreatment with heteropoly acids in γ-valerolactone/water. Bioresour. Technol. 2018, 261, 70–75. [Google Scholar] [CrossRef]
- Lundquist, K.; Kirk, T.K. Acid degradation of lignin IV: Analysis of lignin acidolysis products by gas chromatography, using trimethylsilyl derivatives. Acta Chem. Scand. 1971, 25, 889–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundquist, K.; Ericsson, L. Acid degradation of lignin VI: Formation of methanol. Acta Chem. Scand. 1971, 25, 756–758. [Google Scholar] [CrossRef]
- Lundquist, K.; Lundgren, R. Acid degradation of lignin part VII: The cleavage of ether bonds. Acta Chem. Scand. 1972, 26, 2005–2023. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, S.; Terashima, N.; Ito, T. Chemical structures of sulfuric acid lignin II: Chemical structures of condensation products from arylglycerol-β-aryl ether type structures. Mokuzai Gakkaishi 1981, 27, 216–222. [Google Scholar]
- Hoo, L.H.; Sarkanen, K.V.; Anderson, C.D. Formation of C6C2-enol ethers in the acid-catalyzed hydrolysis of erythro-veratrylglycerol-β-(2-methoxyphenyl) ether. J. Wood Chem. Technol. 1983, 3, 223–243. [Google Scholar] [CrossRef]
- Yokoyama, T.; Matsumoto, Y. Revisiting the mechanism of β-o-4 bond cleavage during acidolysis of lignin. Part 2: Detailed reaction mechanism of a non-phenolic C6-C2 type model compound. J. Wood Chem. Technol. 2010, 30, 269–282. [Google Scholar] [CrossRef]
- Karlsson, O.; Lundquist, K.; Meuller, S.; Westlid, K. On the acidolytic cleavage of arylglycerol β-aryl ethers. Acta Chem.Scand. 1988, B42, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Lundquist, K. Acid degradation of lignin II: Separation and identification of low molecular weight phenols. Acta Chem. Scand. 1970, 24, 889–907. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Lundquist, K. Acid reactions of lignin models of β-5 type. Holzforschung 1999, 53, 39–42. [Google Scholar] [CrossRef]
- Adler, E.; Lundquist, K. Spectrochemical estimation of phenylcoumaran elements in lignin. Acta Chem. Scand. 1963, 17, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Lundquist, K.; Hedlund, K. Acid degradation of lignin V: Degradation products related to the phenylcoumaran type of structure. Acta Chem. Scand. 1971, 25, 2199–2210. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Azarpira, A.; Motagamwala, A.H.; Lu, F.; Ralph, J.; Dumesic, J.A. Lignin monomer production integrated into the γ-valerolactone sugar platform. Energ. Environ. Sci. 2015, 8, 2657–2663. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, T.; Uraki, Y.; Ubukata, M. Easy synthesis of beta-o-4 type lignin related polymers. Org. Biomol. Chem. 2005, 3, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Jiang, T.; Aslam Bhatti, H.; Siddiqui, B.S.; Dixon, S.; Kilburn, J.D. Synthesis of dihydrodehydrodiconiferyl alcohol: The revised structure of lawsonicin. Org. Biomol. Chem. 2010, 8, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lundquist, K.; Holm, K.; Skattebøl, L.; Rosendahl, C.N. Synthesis of lignin models of beta-5 type. Acta Chem. Scand. 1997, 51, 1224–1228. [Google Scholar] [CrossRef]
- Habib, M.; Trajkovic, M.; Fraaije, M.W. The biocatalytic synthesis of syringaresinol from 2,6-dimethoxy-4-allylphenol in one-pot using a tailored oxidase/peroxidase system. ACS Catal. 2018, 8, 5549–5552. [Google Scholar] [CrossRef] [PubMed]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Fraile, J.M.; García, J.I.; Hormigón, Z.; Mayoral, J.A.; Saavedra, C.J.; Salvatella, L. Role of substituents in the solid acid-catalyzed cleavage of the β-o-4 linkage in lignin models. ACS Sustain. Chem. Eng. 2018, 6, 1837–1847. [Google Scholar] [CrossRef]
- Ito, T.; Terashima, N.; Yasuda, S. Chemical structures of sulfuric acid lignin III: Reaction of arylglycerol-β-aryl ether with five percent sulfuric acid. Mokuzai Gakkaishi 1981, 27, 484–490. [Google Scholar]
- Yasuda, S.; Ota, K. Chemical structures of sulfuric acid lignin pt.X.* reaction of syringylglycerol-β-syringyl ether and condensation of syringyl nucleus with guaiacyl lignin model compounds in sulfuric acid. Holzforschung 1987, 41, 59–65. [Google Scholar] [CrossRef]
- Sturgeon, M.R.; Kim, S.; Lawrence, K.; Paton, R.S.; Chmely, S.C.; Nimlos, M.; Foust, T.D.; Beckham, G.T. A mechanistic investigation of acid-catalyzed cleavage of aryl-ether linkages: Implications for lignin depolymerization in acidic environments. ACS Sustain. Chem. Eng. 2014, 2, 472–485. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sener, C.; Gallo, J.M.; Luterbacher, J.S.; Alonso, D.M.; Dumesic, J.A. Solvent effects in acid-catalyzed biomass conversion reactions. Angew. Chem. Int. Ed. Engl. 2014, 53, 11872–11875. [Google Scholar] [CrossRef] [PubMed]
- Sarkanen, K.V.; Hoo, L.H. Kinetics of hydrolysis of erythro-guaiacylglycerol β-(2-methoxyphenyl) ether and its veratryl analogue using HCl and aluminum chloride as catalysts. J.Wood Chem. Technol. 1981, 1, 11–27. [Google Scholar] [CrossRef]
- Jia, S.; Cox, B.J.; Guo, X.; Zhang, Z.C.; Ekerdt, J.G. Hydrolytic cleavage of β-o-4 ether bonds of lignin model compounds in an ionic liquid with metal chlorides. Ind. Eng. Chem. Res. 2011, 50, 849–855. [Google Scholar] [CrossRef]
- Jia, S.; Cox, B.J.; Guo, X.; Zhang, Z.C.; Ekerdt, J.G. Cleaving the β-o-4 bonds of lignin model compounds in an acidic ionic liquid, 1-H-3-methylimidazolium chloride: An optional strategy for the degradation of lignin. ChemSusChem 2010, 3, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Matsumoto, Y. Revisiting the mechanism of β-o-4 bond cleavage during acidolysis of lignin. Part 1: Kinetics of the formation of enol ether from non-phenolic C6-C2 type model compounds. Holzforschung 2008, 62, 164–168. [Google Scholar] [CrossRef]
- Imai, T.; Yokoyama, T.; Matsumoto, Y. Revisiting the mechanism of β-o-4 bond cleavage during acidolysis of lignin IV: Dependence of acidolysis reaction on the type of acid. J.Wood Sci. 2011, 57, 219–225. [Google Scholar] [CrossRef]
- Lin, L.; Nakagame, S.; Yao, Y.; Yoshioka, M.; Shiraishi, N. Liquefaction mechanism of β-o-4 lignin model compound in the presence of phenol under acid catalysis part 2. Reaction behavior and pathways. Holzforschung 2001, 55, 625–630. [Google Scholar] [CrossRef]
- Lin, L.; Yao, Y.; Shiraishi, N. Liquefaction mechanism of β-o-4 lignin model compound in the presence of phenol under acid catalysis part 1. Identification of the reaction products. Holzforschung 2001, 55, 617–624. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Lignans and kauranes from the stems of annona cherimola. J. Chin. Chem. Soc. 1998, 45, 629–634. [Google Scholar] [CrossRef]
- Chang, F.R.; Chao, Y.C.; Teng, C.M.; Wu, Y.C. Chemical constituents from cassytha filiformis II. J.Nat. Prod. 1998, 61, 863–866. [Google Scholar] [CrossRef] [PubMed]








| Reaction Time/min | 20 | 40 | 60 |
|---|---|---|---|
| Product (total isomers) yields (%) | 95.8 | 85.5 | 61.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhao, C.; Yue, F.; Lu, F. Revealing Structural Modifications of Lignin in Acidic γ-Valerolactone-H2O Pretreatment. Polymers 2020, 12, 116. https://doi.org/10.3390/polym12010116
Li S, Zhao C, Yue F, Lu F. Revealing Structural Modifications of Lignin in Acidic γ-Valerolactone-H2O Pretreatment. Polymers. 2020; 12(1):116. https://doi.org/10.3390/polym12010116
Chicago/Turabian StyleLi, Suxiang, Chengke Zhao, Fengxia Yue, and Fachuang Lu. 2020. "Revealing Structural Modifications of Lignin in Acidic γ-Valerolactone-H2O Pretreatment" Polymers 12, no. 1: 116. https://doi.org/10.3390/polym12010116
APA StyleLi, S., Zhao, C., Yue, F., & Lu, F. (2020). Revealing Structural Modifications of Lignin in Acidic γ-Valerolactone-H2O Pretreatment. Polymers, 12(1), 116. https://doi.org/10.3390/polym12010116