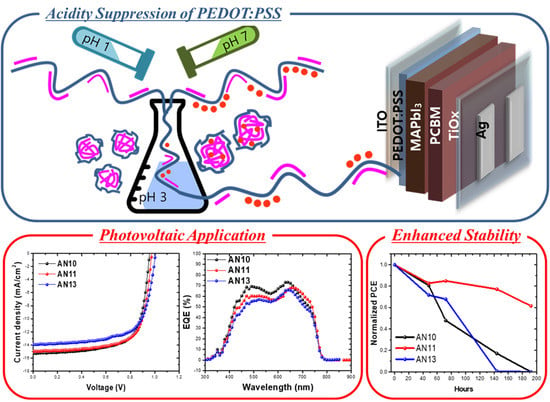
Acidity Suppression of Hole Transport Layer via Solution Reaction of Neutral PEDOT:PSS for Stable Perovskite Photovoltaics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Thin Film and Device Fabrication
2.3. Device Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jaiswal, M.; Menon, R. Polymer electronic materials: A review of charge transport. Polym. Int. 2006, 55, 1371–1384. [Google Scholar] [CrossRef]
- Ho, P.K.H.; Stephen, D.; Thomas; Friend, R.H.; Tessler, N. All-Polymer Optoelectronic Devices. Science 1999, 285, 233. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.C.; Granström, M.; Thomas, D.S.; Petritsch, K.; Friend, R.H. Doped conducting-polymer—Semiconducting-polymer interfaces: Their use in organic photovoltaic devices. Phys. Rev. B 1999, 60, 1854–1860. [Google Scholar] [CrossRef]
- Gustafsson, J.C.; Liedberg, B.; Inganäs, O. In situ spectroscopic investigations of electrochromism and ion transport in a poly (3,4-ethylenedioxythiophene) electrode in a solid state electrochemical cell. Solid State Ion. 1994, 69, 145–152. [Google Scholar] [CrossRef]
- Saghaei, J.; Fallahzadeh, A.; Yousefi, M.H. Improvement of electrical conductivity of PEDOT:PSS films by 2-Methylimidazole post treatment. Org. Electron. 2015, 19, 70–75. [Google Scholar] [CrossRef]
- Greczynski, G.; Kugler, T.; Keil, M.; Osikowicz, W.; Fahlman, M.; Salaneck, W.R. Photoelectron spectroscopy of thin films of PEDOT–PSS conjugated polymer blend: A mini-review and some new results. J. Electron Spectrosc. Relat. Phenom. 2001, 121, 1–17. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef]
- Im, S.; Kim, W.; Cho, W.; Shin, D.; Chun, D.H.; Rhee, R.; Kim, J.K.; Yi, Y.; Park, J.H.; Kim, J.H. Improved Stability of Interfacial Energy-Level Alignment in Inverted Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 18964–18973. [Google Scholar] [CrossRef]
- de Jong, M.P.; van Ijzendoorn, L.J.; de Voigt, M.J.A. Stability of the interface between indium-tin-oxide and poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) in polymer light-emitting diodes. Appl. Phys. Lett. 2000, 77, 2255–2257. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Kim, J.H.; Liang, P.-W.; Williams, S.T.; Cho, N.; Chueh, C.-C.; Glaz, M.S.; Ginger, D.S.; Jen, A.K.-Y. High-Performance and Environmentally Stable Planar Heterojunction Perovskite Solar Cells Based on a Solution-Processed Copper-Doped Nickel Oxide Hole-Transporting Layer. Adv. Mater. 2015, 27, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Johansson, M.; Andersson, M.R.; Hummelen, J.C.; Inganäs, O. Polymer Photovoltaic Cells with Conducting Polymer Anodes. Adv. Mater. 2002, 14, 662–665. [Google Scholar] [CrossRef]
- Snaith, H.J.; Kenrick, H.; Chiesa, M.; Friend, R.H. Morphological and electronic consequences of modifications to the polymer anode ‘PEDOT:PSS’. Polymer 2005, 46, 2573–2578. [Google Scholar] [CrossRef]
- Aernouts, T.; Vanlaeke, P.; Geens, W.; Poortmans, J.; Heremans, P.; Borghs, S.; Mertens, R.; Andriessen, R.; Leenders, L. Printable anodes for flexible organic solar cell modules. Thin Solid Films 2004, 451–452, 22–25. [Google Scholar] [CrossRef]
- Suchand Sangeeth, C.S.; Jaiswal, M.; Menon, R. Correlation of morphology and charge transport in poly(3,4-ethylenedioxythiophene)–polystyrenesulfonic acid (PEDOT–PSS) films. J. Phys. Condens. Matter 2009, 21, 072101. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Yu, G.; Zhang, C.; Menon, R.; Heeger, A.J. Polymer light-emitting diodes with polyethylene dioxythiophene–polystyrene sulfonate as the transparent anode. Synth. Met. 1997, 87, 171–174. [Google Scholar] [CrossRef]
- Brown, T.M.; Kim, J.S.; Friend, R.H.; Cacialli, F.; Daik, R.; Feast, W.J. Built-in field electroabsorption spectroscopy of polymer light-emitting diodes incorporating a doped poly(3,4-ethylene dioxythiophene) hole injection layer. Appl. Phys. Lett. 1999, 75, 1679–1681. [Google Scholar] [CrossRef]
- Ho, P.K.H.; Kim, J.-S.; Burroughes, J.H.; Becker, H.; Li, S.F.Y.; Brown, T.M.; Cacialli, F.; Friend, R.H. Molecular-scale interface engineering for polymer light-emitting diodes. Nature 2000, 404, 481–484. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Yemata, T.A.; Wang, X.; Lim, S.L.; Chin, W.S.; Hippalgaonkar, K.; Xu, J. Enhanced Thermoelectric Performance of PEDOT:PSS Films by Sequential Post-Treatment with Formamide. Macromol. Mater. Eng. 2018, 303, 1700429. [Google Scholar] [CrossRef]
- Xu, S.; Hong, M.; Shi, X.-L.; Wang, Y.; Ge, L.; Bai, Y.; Wang, L.; Dargusch, M.; Zou, J.; Chen, Z.-G. High-Performance PEDOT:PSS Flexible Thermoelectric Materials and Their Devices by Triple Post-Treatments. Chem. Mater. 2019, 31, 5238–5244. [Google Scholar] [CrossRef]
- Xue, Q.; Chen, G.; Liu, M.; Xiao, J.; Chen, Z.; Hu, Z.; Jiang, X.-F.; Zhang, B.; Huang, F.; Yang, W.; et al. Improving Film Formation and Photovoltage of Highly Efficient Inverted-Type Perovskite Solar Cells through the Incorporation of New Polymeric Hole Selective Layers. Adv. Energy Mater. 2016, 6, 1502021. [Google Scholar] [CrossRef]
- Kim, J.-S.; Ho, P.K.H.; Murphy, C.E.; Baynes, N.; Friend, R.H. Nature of Non-emissive Black Spots in Polymer Light-Emitting Diodes by In-Situ Micro-Raman Spectroscopy. Adv. Mater. 2002, 14, 206–209. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Deng, L.-L.; Dai, S.-M.; Wang, X.; Tian, C.-B.; Zhan, X.-X.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Low-cost solution-processed copper iodide as an alternative to PEDOT:PSS hole transport layer for efficient and stable inverted planar heterojunction perovskite solar cells. J. Mater. Chem. A 2015, 3, 19353–19359. [Google Scholar] [CrossRef]
- Chen, L.-M.; Hong, Z.; Li, G.; Yang, Y. Recent Progress in Polymer Solar Cells: Manipulation of Polymer:Fullerene Morphology and the Formation of Efficient Inverted Polymer Solar Cells. Adv. Mater. 2009, 21, 1434–1449. [Google Scholar] [CrossRef]
- Ramasami, P.; Gupta Bhowon, M.; Jhaumeer Laulloo, S.; Li Kam Wah, H. Emerging Trends in Chemical Sciences; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 405–417. [Google Scholar]
- Fan, P.; Zheng, D.; Zheng, Y.; Yu, J. Efficient and stable planar p-i-n perovskite solar cells by doping tungsten compound into PEDOT:PSS to facilitate perovskite crystalline. Electrochim. Acta 2018, 283, 922–930. [Google Scholar] [CrossRef]
- Niu, Q.; Huang, W.; Tong, J.; Lv, H.; Deng, Y.; Ma, Y.; Zhao, Z.; Xia, R.; Zeng, W.; Min, Y.; et al. Understanding the mechanism of PEDOT:PSS modification via solvent on the morphology of perovskite films for efficient solar cells. Synth. Met. 2018, 243, 17–24. [Google Scholar] [CrossRef]
- Wang, Q.; Chueh, C.C.; Eslamian, M.; Jen, A.K.Y. Modulation of PEDOT:PSS pH for efficient inverted perovskite solar cells with reduced potential loss and enhanced stability. ACS Appl. Mater. Interfaces 2016, 8, 32068–32076. [Google Scholar] [CrossRef]
- Tsai, T.-C.; Chang, H.-C.; Chen, C.-H.; Huang, Y.-C.; Whang, W.-T. A facile dedoping approach for effectively tuning thermoelectricity and acidity of PEDOT:PSS films. Org. Electron. 2014, 15, 641–645. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Gevorgyan, S.A.; Tromholt, T.; Andreasen, B.; Krebs, F.C. Stability of Polymer Solar Cells. Adv. Mater. 2012, 24, 580–612. [Google Scholar] [CrossRef] [PubMed]
- Horii, T.; Hikawa, H.; Mochizuki, Y.; Okuzaki, H. Synthesis and Characterization of Highly Conductive PEDOT/PSS Colloidal Gels. Trans. Mater. Res. Soc. Jpn. 2012, 37, 515–518. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, J.E.; Hanley, C.A.; Brennan, L.J.; Lambertini, V.G.; Gun’ko, Y.K. Fabrication of highly transparent and conducting PEDOT:PSS films using a formic acid treatment. J. Mater. Chem. C 2014, 2, 764–770. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.; Jiang, F.; Xu, J.; Liu, E. Effective treatment methods on PEDOT:PSS to enhance its thermoelectric performance. Synth. Met. 2017, 225, 31–40. [Google Scholar] [CrossRef]
- Horii, T.; Li, Y.; Mori, Y.; Okuzaki, H. Correlation between the hierarchical structure and electrical conductivity of PEDOT/PSS. Polym. J. 2015, 47, 695–699. [Google Scholar] [CrossRef]
- Alemu Mengistie, D.; Wang, P.-C.; Chu, C.-W. Effect of molecular weight of additives on the conductivity of PEDOT:PSS and efficiency for ITO-free organic solar cells. J. Mater. Chem. A 2013, 1, 9907–9915. [Google Scholar] [CrossRef]
- Takano, T.; Masunaga, H.; Fujiwara, A.; Okuzaki, H.; Sasaki, T. PEDOT Nanocrystal in Highly Conductive PEDOT:PSS Polymer Films. Macromolecules 2012, 45, 3859–3865. [Google Scholar] [CrossRef]
- Po, R.; Carbonera, C.; Bernardi, A.; Camaioni, N. The role of buffer layers in polymer solar cells. Energy Environ. Sci. 2011, 4, 285–310. [Google Scholar] [CrossRef]
- Rider, D.A.; Harris, K.D.; Wang, D.; Bruce, J.; Fleischauer, M.D.; Tucker, R.T.; Brett, M.J.; Buriak, J.M. Thienylsilane-Modified Indium Tin Oxide as an Anodic Interface in Polymer/Fullerene Solar Cells. ACS Appl. Mater. Interfaces 2009, 1, 279–288. [Google Scholar] [CrossRef]
- Choi, H.; Mai, C.-K.; Kim, H.-B.; Jeong, J.; Song, S.; Bazan, G.C.; Kim, J.Y.; Heeger, A.J. Conjugated polyelectrolyte hole transport layer for inverted-type perovskite solar cells. Nat. Commun. 2015, 6, 7348. [Google Scholar] [CrossRef] [Green Version]
- Crispin, X.; Jakobsson, F.L.E.; Crispin, A.; Grim, P.C.M.; Andersson, P.; Volodin, A.; van Haesendonck, C.; Van der Auweraer, M.; Salaneck, W.R.; Berggren, M. The Origin of the High Conductivity of Poly(3,4-ethylenedioxythiophene)−Poly(styrenesulfonate) (PEDOT−PSS) Plastic Electrodes. Chem. Mater. 2006, 18, 4354–4360. [Google Scholar] [CrossRef]
- Lang, U.; Müller, E.; Naujoks, N.; Dual, J. Microscopical Investigations of PEDOT:PSS Thin Films. Adv. Funct. Mater. 2009, 19, 1215–1220. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Wu, D.; Zhao, Y.; Shi, X.; Wang, J.; Huang, S.; He, G. Highly conductive and uniform graphene oxide modified PEDOT:PSS electrodes for ITO-Free organic light emitting diodes. J. Mater. Chem. C 2014, 2, 4044–4050. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, G.; Lam, C.-S.; Yao, B.; Ho, C.-L.; Liu, L.; Xie, Z.; Wong, W.-Y.; Ding, J.; Wang, L. High-Efficiency Single Emissive Layer White Organic Light-Emitting Diodes Based on Solution-Processed Dendritic Host and New Orange-Emitting Iridium Complex. Adv. Mater. 2012, 24, 1873–1877. [Google Scholar] [CrossRef]
- Yeo, J.-S.; Yun, J.-M.; Kim, D.-Y.; Park, S.; Kim, S.-S.; Yoon, M.-H.; Kim, T.-W.; Na, S.-I. Significant Vertical Phase Separation in Solvent-Vapor-Annealed Poly(3,4-ethylenedioxythiophene):Poly(styrene sulfonate) Composite Films Leading to Better Conductivity and Work Function for High-Performance Indium Tin Oxide-Free Optoelectronics. ACS Appl. Mater. Interfaces 2012, 4, 2551–2560. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; Janssen, R.A.J.; Bastiaansen, J.A.M.; Kiggen, N.M.M.; Langeveld, B.M.W.; van Breemen, A.J.J.M.; de Kok, M.M. Microscopic Understanding of the Anisotropic Conductivity of PEDOT:PSS Thin Films. Adv. Mater. 2007, 19, 1196–1200. [Google Scholar] [CrossRef]
- Skotheim, T.A.; Reynolds, J. Conjugated Polymers: Processing and Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Vitoratos, E.; Sakkopoulos, S.; Dalas, E.; Paliatsas, N.; Karageorgopoulos, D.; Petraki, F.; Kennou, S.; Choulis, S.A. Thermal degradation mechanisms of PEDOT:PSS. Org. Electron. 2009, 10, 61–66. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef] [Green Version]
- Kemerink, M.; Timpanaro, S.; de Kok, M.M.; Meulenkamp, E.A.; Touwslager, F.J. Three-Dimensional Inhomogeneities in PEDOT:PSS Films. J. Phys. Chem. B 2004, 108, 18820–18825. [Google Scholar] [CrossRef]
- Nardes, A.M.; Janssen, R.A.J.; Kemerink, M. A Morphological Model for the Solvent-Enhanced Conductivity of PEDOT:PSS Thin Films. Adv. Funct. Mater. 2008, 18, 865–871. [Google Scholar] [CrossRef]
- Zhang, D.; Qin, J.; Xue, G. An investigation of the electropolymerization of terthiophene in boron fluoride–ethyl ether. Synth. Met. 1999, 100, 285–289. [Google Scholar] [CrossRef]
- Olivares, A.J.; Cosme, I.; Sanchez-Vergara, M.E.; Mansurova, S.; Carrillo, J.C.; Martinez, H.E.; Itzmoyotl, A. Nanostructural Modification of PEDOT:PSS for High Charge Carrier Collection in Hybrid Frontal Interface of Solar Cells. Polymers 2019, 11, 1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aradilla, D.; Casanovas, J.; Estrany, F.; Iribarren, J.I.; Alemán, C. New insights into the characterization of poly(3-chlorothiophene) for electrochromic devices. Polym. Chem. 2012, 3, 436–449. [Google Scholar] [CrossRef]
- Zhao, Q.; Jamal, R.; Zhang, L.; Wang, M.; Abdiryim, T. The structure and properties of PEDOT synthesized by template-free solution method. Nanoscale Res. Lett. 2014, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Geetha, S.; Trivedi, D.C. A new route to synthesize high degree polythiophene in a room temperature melt medium. Synth. Met. 2005, 155, 232–239. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Xu, J.; Song, H.; Lu, B.; Jiang, F.; Zhou, W.; Zhang, G.; Jiang, Q. Facile Fabrication of PEDOT:PSS/Polythiophenes Bilayered Nanofilms on Pure Organic Electrodes and Their Thermoelectric Performance. ACS Appl. Mater. Interfaces 2013, 5, 12811–12819. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1–16. [Google Scholar] [CrossRef]
- Park, J.-S.; Calbo, J.; Jung, Y.-K.; Whalley, L.D.; Walsh, A. Accumulation of Deep Traps at Grain Boundaries in Halide Perovskites. ACS Energy Lett. 2019, 4, 1321–1327. [Google Scholar] [CrossRef]
- Xie, F.; Chen, C.-C.; Wu, Y.; Li, X.; Cai, M.; Liu, X.; Yang, X.; Han, L. Vertical recrystallization for highly efficient and stable formamidinium-based inverted-structure perovskite solar cells. Energy Environ. Sci. 2017, 10, 1942–1949. [Google Scholar] [CrossRef]
- Ying, X.; Liu, Y.; Ling, L.; Xu, M.; Bao, B.; Bao, X.; Pang, A.; Shao, G.; Zhang, Y.; Fang, J.-K. Low-Cost, High-Efficiency, and Efficiency Predictable Small Molecule Organic Dyes with Bis(4-styryl)phenyl Amino Donor for Dye-Sensitized Solar Cells. Sol. RRL 2019, 3, 1970061. [Google Scholar] [CrossRef]
- Brenner, T.M.; Rakita, Y.; Orr, Y.; Klein, E.; Feldman, I.; Elbaum, M.; Cahen, D.; Hodes, G. Conversion of Single Crystalline PbI2 to CH3NH3PbI3: Structural Relations and Transformation Dynamics. Chem. Mater. 2016, 28, 6501–6510. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, M.; Gao, B.; Chen, W.; Li, Q.; Xu, Q.; Dong, S. Preparation of CH3NH3PbI3 thin films with tens of micrometer scale at high temperature. Sci. Rep. 2017, 7, 8458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirayama, M.; Kato, M.; Miyadera, T.; Sugita, T.; Fujiseki, T.; Hara, S.; Kadowaki, H.; Murata, D.; Chikamatsu, M.; Fujiwara, H. Degradation mechanism of CH3NH3PbI3 perovskite materials upon exposure to humid air. J. Appl. Phys. 2016, 119, 115501. [Google Scholar] [CrossRef] [Green Version]








| Acidic: Neutral | VOC (V) | Jsc (mA/cm2) | EQE (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| AN10 | 0.957 | 15.28 | 14.56 | 70 | 10.31 |
| AN11 | 0.975 | 14.98 | 13.69 | 71 | 10.34 |
| AN13 | 1.006 | 13.94 | 12.59 | 68 | 9.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Yi, M.; Jang, W.; Kim, J.K.; Wang, D.H. Acidity Suppression of Hole Transport Layer via Solution Reaction of Neutral PEDOT:PSS for Stable Perovskite Photovoltaics. Polymers 2020, 12, 129. https://doi.org/10.3390/polym12010129
Kim M, Yi M, Jang W, Kim JK, Wang DH. Acidity Suppression of Hole Transport Layer via Solution Reaction of Neutral PEDOT:PSS for Stable Perovskite Photovoltaics. Polymers. 2020; 12(1):129. https://doi.org/10.3390/polym12010129
Chicago/Turabian StyleKim, Minseong, Minji Yi, Woongsik Jang, Jung Kyu Kim, and Dong Hwan Wang. 2020. "Acidity Suppression of Hole Transport Layer via Solution Reaction of Neutral PEDOT:PSS for Stable Perovskite Photovoltaics" Polymers 12, no. 1: 129. https://doi.org/10.3390/polym12010129
APA StyleKim, M., Yi, M., Jang, W., Kim, J. K., & Wang, D. H. (2020). Acidity Suppression of Hole Transport Layer via Solution Reaction of Neutral PEDOT:PSS for Stable Perovskite Photovoltaics. Polymers, 12(1), 129. https://doi.org/10.3390/polym12010129