1. Introduction
Currently, membrane-based separation technologies are known to be efficient for molecular separations, and they are already considered as alternative processes to traditional methods (absorption, distillation, extractive rectification) at the industrial scale, especially for the separation and purification of azeotropic liquid mixtures and for components having close boiling points. This is because membrane separation technologies offer significant energy savings and can give rise to environmentally friendly processes. Pervaporation is one of the promising classical membrane processes for the dehydration of organic substances, especially alcohols with the use of hydrophilic membranes, because of the high water selectivity of the membranes, equipment compactness, and lower energy consumption [
1,
2,
3,
4,
5,
6,
7]. One of the most studied mixtures for dehydration by pervaporation is the isopropanol (i-PrOH)–water system. Isopropanol is an important solvent widely used for production of various chemical reagents and as a substitute for ethanol in areas such as cosmetics, perfumes, household chemicals, disinfectants, repellents, etc. Yet, for industrial application, the solvent must usually be anhydrous. In addition, this alcohol forms an azeotropic mixture with water (12 wt % water–88 wt % isopropanol) [
8], which considerably hinders its dehydration via simple traditional methods of separation that entail a non-environmentally friendly process (addition of a third harmful organic reagent) and a high energy consumption (high temperature or low pressure, expensive equipment). Pervaporation is a promising and perspective technology for this task. Different types of pervaporation membranes (polymeric, inorganic, and composite) were developed for the dehydration of isopropanol [
9,
10,
11,
12]. Polymeric membranes find wider applications and are attractive because of the simplicity of their fabrication and lower price compared to inorganic membranes. The most popular polymer materials for dehydration are green water-soluble polymers, such as polyvinyl alcohol (PVA), chitosan (CS), cellulose, and sodium alginate [
1,
13,
14], because these polymers possess high selectivity to water. However, the use of membranes based on these polymer materials requires additional cross-linking that, as a rule, leads to the decrease of permeation flux [
12]. To improve the transport characteristics, additional bulk and surface modification methods can to be successfully applied, as previously shown [
15,
16,
17,
18,
19,
20].
In this study, the selected material is polyvinyl alcohol, which is widely used for dehydration purposes due to its high water selectivity, good film-forming properties, and economic accessibility [
1,
4,
21,
22]. However, pristine PVA membranes without additional modification and cross-linking exhibit poor stability in aqueous solutions where large swelling is observed. This phenomenon makes it impossible to use them for the separation of dilute solutes.
Earlier studies and literature reviews demonstrated that the application of polyelectrolytes for bulk and surface modification could lead to significant improvement of transport characteristics due to extrinsic and intrinsic charge overcompensation mechanisms and ion pairing, which led to significant changes in free volume and surface hydrophilicity [
23,
24].
One of the modern and versatile methods of membrane surface modification is the layer-by-layer (LbL) assembly technique for the fabrication of ultra-thin defect-free polyelectrolyte (PEL) layers on the surface of the polymer membrane [
25]. This method consists of sequential alternating adsorption of polycations and polyanions on a membrane surface and allows one to control the thickness and the surface properties (for example, hydrophilicity, etc.) of membranes. Varying modification conditions such as the number of PEL layers, types of PEL, their ionic strength, and pH of PEL solutions permits flexibly improving the pervaporation characteristics of membranes [
26,
27,
28]. The LbL assembly technique was firstly explored by Hong and Decher in 1991 and reported by Iler in 1996 [
25]. Surface modification by LbL deposition of PEL can be carried out via various methods such as spray-LbL, spin-LbL, and dip-LbL. It is one of the common simple ways for modifying polymer membranes due to the formation of denser, thicker, and smoother PEL multilayers [
25]. The LbL modification by PEL of PVA polymer is applied in different fields of science and technology [
29,
30,
31]. In Reference [
29], a novel high-flux membrane for forward osmosis (FO) was prepared via LbL deposition of three PEL bilayers (chitosan (CS)/polyacrylic acid (PAAc)) on the developed membrane substrate based on a nanocomposite of polyvinyl alcohol (PVA) and montmorillonite clay. The developed FO membranes were tested in deionized water (DI) and synthetic wastewater. It was shown that the application of LbL surface modification of the prepared substrate allowed the achievement of low salt leakage. LbL coating of different bilayer numbers (5, 10, 20) of poly (diallyldimethylammonium chloride)/polystyrene sulfonic acid sodium salt) was also applied for the development of stable anion exchange KOH-doped PVA membranes cross-linked with glutaraldehyde (GA) or poly(ethylene glycol) diglycidyl ether (PEDGE) [
30]. It was determined that the ionic conductivity of the membranes increased with the increase in the number of bilayers, and the multilayered film was stable against fuel cell operating conditions in terms of pH. Thus, the application of the LbL technique for anion exchange membranes makes it possible to improve their characteristics and become promising membranes for fuel cell applications. In Reference [
31], LbL deposition using 2-anthraquinone sulfonate (2-AQS), 2,6-AQS, and 2,7-AQS with polyethyleneimine (PEI) was used for modification of the surface of poly(vinylalcohol-
co-ethylene) (PVA-
co-PE) nanofiber composite membranes to develop membranes with photoinduced self-cleaning functions. The deposition of only one layer of 2,6-AQS was able to provide the expected photoinduced self-cleaning properties, while the increase in the number of bilayers led to an adverse effect on the decomposition efficiency.
One of the most commonly used cationic polyelectrolytes is poly(allylamine hydrochloride) (PAH) due to its high affinity for water, because of the presence of amine hydrochloride functional groups [
32,
33]. Polyelectrolyte PAH was already used as a noncovalent agent to functionalize multi-walled carbon nanotubes (MWCNT) for better dispersion into the PVA matrix [
34]. Nanocomposite membranes based on PVA/MWCNT–PAH were prepared for pervaporation dehydration of isopropanol, and they were shown to possess improved transport properties compared to the pristine membrane. Namboodiri et al. [
32,
33] developed new dense membranes based on mixtures of poly(allylamine hydrochloride)–poly(vinyl alcohol) cross-linked by glutaraldehyde (GA) for the pervaporation dehydration of various organic solvents such as ethanol, methanol, acetone, and isopropanol. They incorporated PVA into the PAH matrix at various formulation ratios to get improved membrane characteristics, such as flexibility and stability.
Our previous studies [
23,
35] showed that many parameters could be used to tune the PEL effect on the transport characteristics of the PVA membranes, such as the selected pair of polycation–polyanion, the order of their deposition via the LbL deposition technique, the number of cycles, and additional bulk modification (introduction of PAH or CS). Thus, a PVA–fullerenol membrane modified by PAH (4.7 wt %) exhibited the best transport properties for the dehydration of isopropanol after an additional surface modification by 10 bilayers of poly(sodium 4-styrenesulfonate) (PSS)/PAH, which led to an increase in the permeation flux (0.29 kg/(m
2h)) with a high level of selectivity (water content in the permeate was 98.4 wt %) [
23]. PAH was introduced into the PVA matrix in order to improve the dispersion of fullerenol in the membrane matrix, as well as the adhesion of the PEL layers for surface modification. It was also observed that the deposition of PSS/PAH layers onto a non-porous selective PVA layer promoted the stability of PEL layers during pervaporation (nano-sized polyelectrolyte layers were not washed away). On the other hand, the use of porous substrate based on polyethyleneterephthalate with polyacrylonitrile resulted in a limitation of the stability in water of the deposited PSS/PAH nanolayers (up to 20 wt % water in the feed) and the limitation of the number of PEL bilayers (from 15 bilayers) [
36]. It should be noted that, in References [
23,
35], the effect of PAH introduction into the PVA matrix and the interaction nature between PAH and PVA were not provided. Moreover, the selectivity properties of the best PVA–fullerenol (5%)–PAH/LbL-10 membrane could be improved (98.4%).
The aim of this work was to study dense and supported pervaporation PVA-based membranes modified with poly(allylamine hydrochloride) (PAH) and PSS/PAH top nanolayers. The transport properties of the prepared membranes based on PVA–PAH were studied by pervaporation of an isopropanol–water mixture. Two main points were investigated: (1) the role of the polyelectrolyte PAH on properties of the polymer matrix studied by FTIR spectroscopy, scanning electron microscopy (SEM), atomic force microscopy (AFM), pervaporation, and contact-angle measurements, and (2) the impact of the commercial porous substrates based on polyacrylonitrile (PAN) and aromatic polysulfone amide (UPM-20®) investigated by SEM, AFM, the standard porosimetry method, contact-angle measurements, and ultrafiltration for understanding the mass transfer in the pervaporation of isopropanol dehydration.
In order to assess the promising application of the PAN-supported membranes modified by PEL, its transport properties were studied at different temperatures (20, 30, 45, 60 °C) during pervaporation dehydration of the i-PrOH (20 wt % water), compared with the commercial analog PERVAPTM 1201. Stability of top nanolayers deposited via the LbL technique on the surface of the PAN-supported membrane was studied by contact-angle measurements and SEM.
4. Conclusions
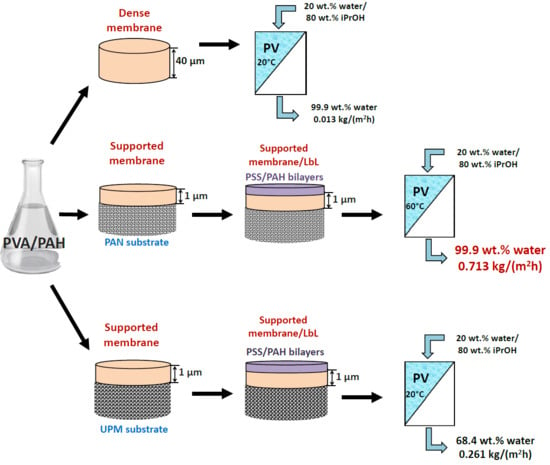
In this work, the effect of the introduction of the widely used cationic polyelectrolyte poly(allylamine hydrochloride) (PAH) into a PVA matrix for understanding the mechanism of mass transfer in pervaporation dehydration was studied. It was shown that the modification of PVA by PAH led to a significant change in the physical membrane properties; it changed the internal and surface morphology of membranes, increased the hydrophilicity of the surface, and led to a change in the structural characteristics of the membranes. These phenomena significantly affected the transport properties of the developed PVA-based membranes and caused a ~2-fold increase in permeation flux while preserving the high water content in the permeate (99.9 wt %) for the dense PVA–PAH membrane compared to the pristine dense PVA membrane during the separation of an isopropanol–water (80/20 wt %) mixture by pervaporation at 20 °C.
Additionally, the influence of the nature of the used commercial porous substrates (PAN and UPM-20) for the preparation of supported PVA-based membranes with bulk (the introduction of PAH into the PVA matrix) and surface (the deposition of the PSS/PAH bilayers) modifications for pervaporation dehydration of i-PrOH (20 wt % water) was studied.
It was found that the application of PAN substrate for PVA-based membranes with or without 10 PEL bilayers led to much higher water content in the permeate and lower permeation flux (~4-fold) compared to the application of the UPM-20 substrate during pervaporation of an i-PrOH (80 wt %)/water (20 wt %) mixture at 20 °C. It should be underlined that the effect of surface modification via LbL deposition of 10 bilayers for supported PVA–PAH membranes was quite different depending on the used substrate. Specifically, the coating of 10 bilayers (PSS/PAH) on the surface of the PVA–PAHUPM membrane led to a significant decrease in water content in the permeate with a slight increase of permeation flux compared to the PVA–PAH membrane without surface modification on UPM-20 (PVA–PAHUPM). For the PAN-supported membrane, there was another trend; both membrane transport parameters were improved after the surface modification with 10 bilayers (PSS/PAH). Thus, it was demonstrated that the transport characteristics of the supported membranes depended significantly on the choice of the commercial porous substrate (UPM-20 and PAN) because of differences in the chemical nature of the substrate material, pore size, and porosity.
Based on the obtained results, it could be concluded that a supported membrane with a thin selective layer based on a polymer blend of PVA–PAH deposited on a porous PAN substrate and modified with 10 PEL bilayers via LbL deposition (PVA–PAH/LbL-10PAN membrane) exhibited highly selective properties with respect to water (≥99 wt % water in the permeate) at different temperatures (20, 30, 45, 60 °C). Moreover, this membrane also had a permeation flux ~4.5-fold higher than that of the commercial analog PERVAPTM 1201 (Sulzer) with the same selectivity level for the pervaporation dehydration of i-PrOH (20 wt % water) at 60 °C. This developed membrane is promising for industrial application in the dehydration of organic solvents.