Preparation of Polyaniline/Emulsion Microsphere Composite for Efficient Adsorption of Organic Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
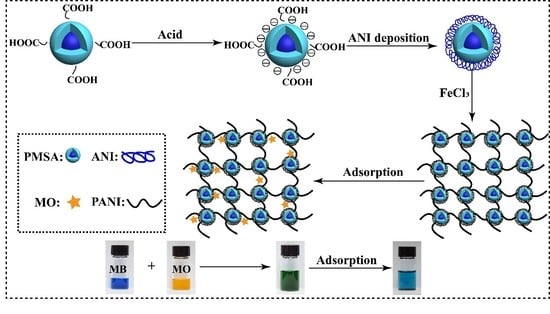
2.2. Preparation of PANI/PSMA
2.3. Adsorption Experiment
2.4. Selective Adsorption
2.5. Adsorption–Desorption Recycling Experiment
2.6. Adsorption in Simulated Industrial Wastewater (SIW)
2.7. Measurements and Characterizations
3. Results
3.1. The Structural Characterization of PANI/PSMA
3.2. Adsorption Characteristics of PANI/PSMA for MO
3.2.1. Effects of pH
3.2.2. Effects of the Dye Type
3.2.3. Adsorption Kinetics
3.2.4. Adsorption Isotherms
3.2.5. Adsorption Thermodynamics
3.2.6. Selective Adsorption Experiments
3.2.7. Adsorption–Desorption Recycling Experiments
3.2.8. EDS Analysis before and after Adsorption
3.2.9. The Adsorption of SIW
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afkhami, A.; Moosavi, R. Adsorptive removal of congo red, a carcinogenic textile dye, from aqueous solutions by maghemite nanoparticles. J. Hazard. Mater. 2010, 174, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Gad-allah, T.A.; Kato, S.; Satokawa, S.; Kojima, T. Treatment of synthetic dyes wastewater utilizing a magnetically separable photocatalyst (TiO2/SiO2/Fe3O4): Parametric and kinetic studies. Desalination 2009, 244, 1–11. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Fu, Y.Q.; Jiang, J.H.; Xiao, L.; Zeng, G.M. Preparation, characterization and dye adsorption properties of γ-Fe2O3/SiO2/chitosan composite. Appl. Surf. Sci. 2011, 258, 1337–1344. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B 2009, 87, 105–145. [Google Scholar]
- Sharma, P.; Kaur, H.; Sharma, M.; Sahore, V. A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environ. Monit. Assess. 2011, 183, 151–195. [Google Scholar] [CrossRef]
- Hosseini, S.; Khan, M.A.; Malekbala, M.R.; Cheah, W.; Choong, T.S.Y. Carbon coated monolith, a mesoporous material for the removal of methyl orange from aqueous phase: Adsorption and desorption studies. Chem. Eng. J. 2011, 171, 1124–1131. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hassan, M.; Gomes, V.G. Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal., A 2015, 489, 1–16. [Google Scholar] [CrossRef]
- Rakkesh, R.A.; Durgalakshmi, D.; Balakumar, S. Efficient sunlight-driven photocatalytic activity of chemically bonded GNS-TiO2 and GNS-ZnO heterostructures. J. Mater. Chem. C. 2014, 2, 6827–6834. [Google Scholar] [CrossRef]
- Reddy, K.R.; Gomes, V.G.; Hassan, M. Carbon functionalized TiO2 nanofibers for high efficiency photocatalysis. Mater. Res. Express. 2014, 1, 015012. [Google Scholar] [CrossRef]
- Kamegawa, T.; Seto, H.; Matsuura, S.; Yamashita, H. Preparation of hydroxynaphthalene-modified TiO2 via formation of surface complexes and their applications in the photocatalytic reduction of nitrobenzene under visible-light irradiation. ACS Appl. Mater. Interfaces. 2012, 4, 6635–6639. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, M.R.; Anirudhan, T.S. The kinetics and thermodynamics of sorption of chromium(VI) onto the iron(III) complex of a carboxylated polyacrylamide-grafted sawdust. Ind. Eng. Chem. Res. 2001, 40, 2693–2701. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Wu, J.; Dai, G. Isotherm, thermodynamic, kinetics and adsorption mechanism studies of methyl orange by surfactant modified silkworm exuviae. J. Hazard. Mater. 2011, 192, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, J.C.; He, B.; Zhang, F.A.; Li, H.B. Peach gum for efficient removal of methylene blue and methyl violet dyes from aqueous solution. Carbohydr. Polym. 2014, 101, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Bai, Z.; Yong, Z.; Wang, B.J.; Luo, W.Q. Fabrication of chitosan microspheres for efficient adsorption of methyl orange. Chin. J. Chem. Eng. 2018, 26, 657–666. [Google Scholar] [CrossRef]
- Daoud, M.; Benturki, O.; Girods, P.; Donnot, A.; Fontana, S. Adsorption ability of activated carbons from phoenix dactylifera rachis and ziziphus jujube stones for the removal of commercial dye and the treatment of dyestuff wastewater. Microchem. J. 2019, 148, 493–502. [Google Scholar] [CrossRef]
- Iida, Y.; Kozuka, T.; Tuziuti, T.; Yasui, K. Sonochemically enhanced adsorption and degradation of methyl orange with activated aluminas. Ultrasonics 2004, 42, 635–639. [Google Scholar] [CrossRef]
- Yao, Y.J.; He, B.; Xu, F.F.; Chen, X.F. Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem. Eng. J. 2011, 170, 82–89. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. Polyaniline/single-wall carbon nanotube (pani/swcnt) composites for high performance supercapacitors. Electrochim. Acta 2006, 52, 1721–1726. [Google Scholar] [CrossRef]
- Bhaumik, M.; Mccrindle, R.; Maity, A. Efficient removal of Congo red from aqueous solutions by adsorption onto interconnected polypyrrole–polyaniline nanofibres. Chem. Eng. J. 2013, 228, 506–515. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Zhang, F.A.; Yu, C.B.; Wei, Z.B. Multicarboxylic hyperbranched polyglycerol modified SBA-15 for the adsorption of cationic dyes and copper ions from aqueous media. Appl. Surf. Sci. 2012, 258, 5291–5298. [Google Scholar] [CrossRef]
- Aliabadi, R.S.; Mahmoodi, N.O. Synthesis and characterization of polypyrrole, polyaniline nanoparticles and their nanocomposite for removal of azo dyes; sunset yellow and congo red. J. Cleaner Prod. 2018, 179, 235–245. [Google Scholar] [CrossRef]
- Yan, B.; Chen, Z.H.; Cai, L.; Chen, Z.M.; Fu, J.W.; Xu, K. Fabrication of polyaniline hydrogel: Synthesis, characterization and adsorption of methylene blue. Appl. Surf. Sci. 2015, 356, 39–47. [Google Scholar] [CrossRef]
- Janaki, V.; Vijayaraghavan, K.; Oh, B.T.; Lee, K.J.; Muthuchelian, K.; Ramasamy, A.K.; Kamala-Kannan, S. Starch/polyaniline nanocomposite for enhanced removal of reactive dyes from synthetic effluent. Carbohydr. Polym. 2012, 90, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Wen, Y.Q.; Ge, H.L.; Sun, Z.W. Simple fabrication of full color colloidal crystal films with tough mechanical strength. Macromol. Chem. Phys. 2010, 207, 596–604. [Google Scholar] [CrossRef]
- Bhadra, S.; Chattopadhyay, S.; Singha, N.K.; Khastgir, D. Improvement of conductivity of electrochemically synthesized polyaniline. J. Appl. Polym. Sci. 2008, 108, 57–64. [Google Scholar] [CrossRef]
- Aghajani, K.; Tayebi, H.A. Synthesis of SBA-15/PAni mesoporous composite for adsorption of reactive dye from aqueous media: RBF and MLP networks predicting models. Fibers Polym. 2017, 18, 465–475. [Google Scholar] [CrossRef]
- Pandimurugan, R.; Thambidurai, S. Synthesis of seaweed-ZnO-PANI hybrid composite for adsorption of methylene blue dye. J. Environ. Chem. Eng. 2016, 4, 1332–1347. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Synthesis of uniform polyaniline nanofibers through interfacial polymerization. Materials 2012, 5, 1487–1494. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Zhang, J.; Zhu, Y.; Wang, S.; Wang, X.; Lv, K. Novel polymer material for efficiently removing methylene blue, Cu(II) and emulsified oil droplets from water simultaneously. Polymers 2018, 10, 1393. [Google Scholar] [CrossRef] [Green Version]
- Mi, F.L.; Wu, S.J.; Chen, Y.C. Combination of carboxymethyl chitosan-coated magnetic nanoparticles and chitosan-citrate complex gel beads as a novel magnetic adsorbent. Carbohydr. Polym. 2015, 131, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Duan, Y.; Zhou, L. Multi-carboxylic magnetic gel from hyperbranched polyglycerol formed by thiol-ene photopolymerization for efficient and selective adsorption of methylene blue and methyl violet dyes. J. Colloid Interface Sci. 2018, 529, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yang, Z.; Cui, L.; Li, Y.; Ngo, H.H.; Wang, Y.; Wei, Q.; Ma, H.; Yan, L.; Du, B. Fabrication of hyperbranched polyamine functionalized graphene for high-efficiency removal of Pb(II) and methylene blue. Chem. Eng. J. 2016, 287, 545–556. [Google Scholar] [CrossRef]
- Deng, S.; Xu, H.; Jiang, X.; Yin, J. Poly(vinyl alcohol) (PVA)-enhanced hybrid hydrogels of hyperbranched poly(ether amine) (hPEA) for selective adsorption and separation of dyes. Macromolecules 2013, 46, 2399–2406. [Google Scholar] [CrossRef]
- Singh, S.; Barick, K.C.; Bahadur, D. Fe3O4 embedded ZnO nanocomposites for the removal of toxic metal ions, organic dyes and bacterial pathogens. J. Mater. Chem. A 2013, 1, 3325–3333. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, B.; Wang, H.; Zhao, F.; Chen, Y.; Sun, Q. Facile crosslinking synthesis of hyperbranch-substrate nanonetwork magnetite nanocomposite for the fast and highly efficient removal of lead ions and anionic dyes from aqueous solutions. RSC Adv. 2016, 6, 67057–67071. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Rodríguez-López, M.I.; Fortea, M.I.; Lucas-Abellán, G.; Mercader-Ros, M.T.; López-Miranda, S.; Gómez-López, V.M.; Semeraro, P.; Cosma, P.; Fini, P.; et al. Adsorption properties of beta- and hydroxypropyl-beta-cyclodextrins cross-linked with epichlorohydrin in aqueous solution. A sustainable recycling strategy in textile dyeing process. Polymers 2019, 11, 252. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Lu, M.; Wang, D.; Song, Y.; Zhou, L. Versatile magnetic gel from peach gum polysaccharide for efficient adsorption of Pb2+ and Cd2+ ions and catalysis. Carbohydr. Polym. 2017, 181, 785–792. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Moszyński, D.; Mijowska, E. Adsorption of anionic azo-dyes from aqueous solutions onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. J. Colloid Interface Sci. 2017, 496, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Johari, I.S.; Yusof, N.A.; Haron, M.J.; Nor, S.M.M. Preparation and characterization of poly(ethyl hydrazide) grafted oil palm empty fruit bunch for removal of Ni(II) ion in aqueous environment. Polymers 2013, 5, 1056–1067. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Meng, D.; Zhou, L. Facile synthesis of low-cost magnetic biosorbent from peach gum polysaccharide for selective and efficient removal of cationic dyes. Int. J. Biol. Macromol. 2018, 107, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Okesolaa, B.O.; Smith, D.K. Versatile supramolecular pH-tolerant hydrogels which demonstrate pH dependent selective adsorption of dyes from aqueous solution. Chem. Commun. 2013, 49, 11164–11166. [Google Scholar] [CrossRef] [PubMed]
- Li, D.P.; Zhang, Y.R.; Zhao, X.X.; Zhao, B.X. Magnetic nanoparticles coated by aminoguanidine for selective adsorption of acid dyes from aqueous solution. Chem. Eng. J. 2013, 232, 425–433. [Google Scholar] [CrossRef]











| Qe-exp (mg/g) | Pseudo-Second-Order | Pseudo-First-Order | Intraparticle Diffusion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t/Qt = 1/K2Qe2 + t/Qe | ln(Qe − Qt) = lnQe − K1t | Qt = Kit0.5 + C | ||||||||
| Qe-cal (mg/g) | K2 (g/mg·min) | R2 | Qe-cal (mg/g) | K1 (1/min) | R2 | Ki | C | R2 | ||
| 20 | 79.37 ± 0.21 | 81.301 | 0.00275 | 0.9995 | 36.270 | 0.0345 | 0.9575 | 2.522 | 46.937 | 0.8077 |
| 30 | 112.10 ± 2.96 | 116.414 | 0.00114 | 0.9984 | 56.940 | 0.0185 | 0.9580 | 3.938 | 59.453 | 0.9173 |
| Isotherm Model | Parameters | MO |
|---|---|---|
| Langmuir: Ce/Qe = Ce/Qm + 1/QmKL | Qm/(mg·g−1) | 147.929 |
| KL/(L·mg−1) | 0.180 | |
| R2 | 0.9999 | |
| Freundlich: lnQe = lnKF + bFlnCe | KF/(mg·g−1) | 93.785 |
| bF | 0.138 | |
| R2 | 0.9544 | |
| Temkin: Qe = BlnKT + BlnCe | KT/(L·mg−1) | 13266.535 |
| B/(KJ−2·mol−2) | 11.367 | |
| R2 | 0.9786 |
| Temperature (K) | ∆G0 (kJ·mol−1) | ∆S0 (J·mol−1·K−1) | ∆H0 (KJ·mol−1) |
|---|---|---|---|
| 298 | −4.996 | 16.823 | 0.0168 |
| 308 | −5.164 | ||
| 318 | −5.333 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Song, L.; Du, L.; Gao, P.; Liang, N.; Wu, S.; Minami, T.; Zang, L.; Yu, C.; Xu, X. Preparation of Polyaniline/Emulsion Microsphere Composite for Efficient Adsorption of Organic Dyes. Polymers 2020, 12, 167. https://doi.org/10.3390/polym12010167
Liu Y, Song L, Du L, Gao P, Liang N, Wu S, Minami T, Zang L, Yu C, Xu X. Preparation of Polyaniline/Emulsion Microsphere Composite for Efficient Adsorption of Organic Dyes. Polymers. 2020; 12(1):167. https://doi.org/10.3390/polym12010167
Chicago/Turabian StyleLiu, Yuanli, Liushuo Song, Linlin Du, Peng Gao, Nuo Liang, Si Wu, Tsuyoshi Minami, Limin Zang, Chuanbai Yu, and Xu Xu. 2020. "Preparation of Polyaniline/Emulsion Microsphere Composite for Efficient Adsorption of Organic Dyes" Polymers 12, no. 1: 167. https://doi.org/10.3390/polym12010167
APA StyleLiu, Y., Song, L., Du, L., Gao, P., Liang, N., Wu, S., Minami, T., Zang, L., Yu, C., & Xu, X. (2020). Preparation of Polyaniline/Emulsion Microsphere Composite for Efficient Adsorption of Organic Dyes. Polymers, 12(1), 167. https://doi.org/10.3390/polym12010167