3.1. Structure and Thermal Stability: Effect of MWCNT Content on Interactions between Molecular Components and Morphology
The hydrogel matrix proposed in this work is formed via copolymerization of acrylamide and bis-acrylamide, with the optimal composition decided based on work reported by Sun et al. [
22]
, and by a previous study carried out in our group [
31]. Briefly, the reaction is vinyl addition polymerization via a free radical-generating system initiated by ammonium persulfate and TEMED. TEMED accelerates the rate of formation of radicals from persulfate that reacts with acrylamide monomers (resulting in radicals that react with neutral monomer and form a polymer). The extending polymer chains are randomly cross-linked by
N,
N′-methylenebisacrylamide (
Figure 1; blue), resulting in stiff gels. In addition, in the presence of alginate and calcium chloride, two types of cross-linked polymer blocks are formed: ionically cross-linked alginate (
Figure 1; red), and covalently cross-linked polyacrylamide. In an aqueous solution, guluronic acid units in alginate chains form ionic crosslinks through Ca
2+ coordination. By contrast, in a polyacrylamide hydrogel, the polyacrylamide chains form a network by covalent crosslinks. There also exist alginate-polyacrylamide hybrid fractions formed when the two types of polymer networks are joined by covalent crosslinks (
Figure 1; green).
The most problematic aspect of these combined materials is the homogenous incorporation of MWCNT within the polymer. This is because of the tendency of MWCNT to agglomerate under electrostatic forces. Magnetic stirring, sonication, and other homogenization methods are not effective. Using surfactants for MWCNT dispersion in the solution of monomers is more challenging, affects the rate of polymerization, and ultimately the chemical composition of hydrogels, their mechanical strength, and conductivity. As compared to our previous study [
31], Nafion, acting as a surfactant in an aqueous suspension of MWCNT, has been replaced with the viscous solution of sodium alginate. The alginate can both participate in the cross-linking and stabilize the MWCNT in water. Based on the chemical structure of alginate, we can assume that improvement of MWCNT dispersion is related to the modification of the electrical surface charge of MWCNT, defined as zeta-potential (unlike Nafion, which is composed of both hydrophobic and hydrophilic fractions that are typical for the surfactant structure) [
30]. This potential is often related to an electric surface potential (
EElec) and contributes to the total surface energy,
Etot =
EVW +
EElec.
Etot depends on the sum of attractive and repulsive forces between particles, which are both dependent on an inter-particle distance. The London-van der Waals contribution for two particles of the same material (
EVW) is always attractive, thus promoting the aggregation of suspended particles. On the contrary, the repulsive component,
EElec, is related to the formation of an electric double-layer on the particle surface when immersed in a polar solvent. For example, in an aqueous suspension of functionalized MWCNT, negative charge is developed on the particle surface during ionization of the oxygen-rich functionalities (e.g., -COOH). Because of the surface charge, an electrostatic potential is created in the proximity of the nanoparticle, and a concentrated layer of counter ions, known as the Stern layer, is formed. As a result, the zeta potential at the MWCNT surface exceeds the total interaction potential barrier (
Etot), and at a given distance, the particle repulsion is stronger and more attractive than the van der Waals forces, resulting in the formation of homogeneous suspensions [
32,
33]. With this, the carbon nanotubes could be well-dispersed even at very high content without the addition of Nafion.
Figure 2 shows a thermal gravimetric scan of a pure hydrogel (A) and an MWCNT-reinforced hydrogel at two differing MWCNT contents (8 mg mL
−1 and 36 mg mL
−1) plotted with the first derivative of weight loss (in %) as the function of applied temperature. The same tests were done for newly-prepared materials and the same films after a long-term electrochemical charge-discharge scanning (accelerated degradation). The latest was used to analyze possible chemical changes related to electrochemical degradation. All signals acquired below 200 °C are related to the removal of residual water. Regarding this, the hydrogel with the highest MWCNT content (
Figure 2C) showed a negligible amount of water after drying. This is related to the fact that a significant weight fraction of this material is occupied by MWCNT, not by hydrophilic polymers. For the pure hydrogel (
Figure 2A), an initial decomposition temperature (IDT) is detected at 221 °C and the final decomposition temperature (FDT) at 417 °C, resulting in 35% weight loss. The thermal degradation of polyacrylamide occurs in three pyrolysis phases. The first signal at IDT is attributed to the degradation of the pure and not cross-linked acrylamide monomer. Afterward, the decomposition of non-cross-linked polyacrylamide has a peak maximum at 264 °C. The main decomposition of the polyacrylamide cross-linked with
N,
N′-methylenebisacrylamide showed an onset temperature at 305 °C and endset at 440 °C. In the temperature range 221–310 °C, one ammonia molecule is liberated for every two amide groups, resulting in the formation of imide [
22]. Subsequently, thermal degradation of imides and breaking of the polymer backbone occurs at higher temperatures. As discussed in previous work [
22], pure alginate (not cross-linked with acrylamides) has two pyrolysis stages: the first thermal degradation process takes place in the temperature range 225–300 °C. The weight loss in the first stage is attributed to the degradation of the carboxyl groups (leading to CO
2 release). The second stage occurred at much higher temperatures (650–740 °C) and corresponds to depolymerization resulting in a carbonaceous residue. Since the first step of decomposition of alginate overlap with signals related to acrylamide monomers, chemical analysis from the degradation of hybrid gels is difficult. One can observe that the pure alginate-polyacrylamide hybrid gel (
Figure 2A) clearly shows pyrolysis stages shifted from locations of single networks [
22]. This qualitatively demonstrates the formation of new covalent bonds between alginate and polyacrylamide.
MWCNT-embedded hydrogels (
Figure 2B,C) exhibit improved thermal stability in comparison to pure hydrogels. This is manifested by the shift of decomposition temperature of the main polymer blend from 350 °C (
Figure 2A—pure hydrogel) to 370 °C. The percent weight loss also decreases with increasing MWCNT content, especially for the highest carbon loading (
Figure 2C). The higher resistance to heat in the presence of the MWCNT reinforced within the polyacrylamide/alginate network can be also related to weak chemical interactions between the MWCNT and the different reaction sites of both polymers [
27,
29]. For example, the latest study suggested that carboxyl groups present on the MWCNT surface can react with hydroxyl functionalities of alginate, resulting in hydrogen bridging [
27]. These molecular interactions are believed to contribute to both better thermal stability of the MWCNT/polymer hybrid, and also to improved dispersion of the MWCNT in the solution of monomers and within the hydrogel itself. More probably, this chemical interaction is based on the esterification reaction and involves the mentioned carboxylic moieties from oxidized carbon and the hydroxyl groups of alginates. Nevertheless, this reactivity requires significant oxidization of the MWCNT surface (high content of -COOH), which was not found in the present study. The surface elemental analysis of MWCNT using an X-ray photoelectron did not show a significant difference in oxygen content (related to -COOH, -OH, or other oxygen-containing functional groups on the MWCNT surface; data not included) for as-obtained (unwashed) and the acid-washed MWCNT (3 M HNO
3, reflux 1 h). Thus, improved dispersion of carbon within the polymer observed in this work can be assumed as the combined effects of electrostatic interactions (predominant) and a minor contribution from the chemical bond formation between alginate and carboxylic groups present on the MWCNT surface.
Figure 3 demonstrates the FTIR spectrum of pure and MWCNT-containing hydrogels with distinctive signals assigned to acrylamide and alginate polymers. The FTIR spectra for all MWCNT-embedded hydrogels were similar, and corresponded to hydrogel signals, except an intensity of peaks decreased with increasing MWCNT content. FTIR showed only signals related to polymers, which were not influenced by the presence of MWCNT at any loading. Briefly, peaks at 1113 and 1350 cm
−1 corresponded to the C-O stretch and at 1181 cm
−1 were assigned to the C-N stretch, together with the N-H vibration at 2940 cm
−1. The vibrations at 1316, 1401, 1462, 2762, and 2855 cm
−1 corresponded to the C-H bonding, arising from amines and amides present in the hydrogel structure. The band at 1606 cm
−1 was related to the C=C vibration and the band at 1679 cm
−1 was a signal of unreacted carbon double bonds from acrylamide and bis-acrylamide monomers. The amide stretch was also observed at 3442 cm
−1 and the -OH stretch at 3178 cm
−1. In summary, FTIR did not demonstrate chemical interactions between MWCNT and organic fraction. This is presumably due to a very low carbon content per total hydrogel mass, calc. 2.79 wt.% of MWCNT for hydrogels containing 360 mg of carbon.
3.2. Resilience—Mechanical Analysis
Tensile characteristics of pure hydrogel and its combinations with MWCNT are shown in
Figure 4. They were used to explore a maximum elongation length before mechanical failure (
Figure 4A–D) and tensile strength acquired from the load sensor attached to one end of the assembly (
Figure 4, bottom). With an adjustment for area difference (thickness of films was relatively similar for all samples), pure hydrogel tested at a maximum of 22.3 kPa (1.34 N), at a displacement of 271 mm. For the hydrogel with the highest MWCNT content (36 mg mL
−1), the applied sensor load was 28.8 kPa (1.76 N), at the displacement of 77 mm. Overall, the stretch modulus is sixteen times higher than that of the initial length for pure, and almost five times for MWCNT-embedded samples. In trend, an increase of MWCNT correlates to a general increase in tensile strength, with a more rapid decline in stretch modulus. A total force resistance of electrodes synthesized in this work was lower, yet resilience to deformation and stretch modulus held similar (17–21 factor) as compared to pure hydrogel with a similar composition as reported by others [
22]. Although hydrogels fabricated in this work demonstrated lower force resistance to elongation, the comparison with the referred literature can be only qualitative since the results strongly depend on the thickness and geometry of the sample [
22], the size of mounting clamps, as well as on the quality of the clamp (e.g., clamps with a sharp, uneven edge cause the material to be cut through faster). Joddar and colleagues reported the MWCNT embedded in alginate hydrogel (without acrylamide) at a very low load content [
29]. The resilience ranges from 103.5 kPa for the pure hydrogel, 79.5 kPa (1 mg mL
−1 MWCNT), 62 (3 mg mL
−1 MWCNT), and 18 kPa (5 mg mL
−1 MWCNT), with the decrease of total strength and stretch modulus with increasing MWCNT content observed. This conclusion contradicted their early central hypothesis that increasing the MWCNT content would increase the stiffness of the alginate gel [
27]. This could be related to the weak van der Waals forces occurring between the MWCNT and alginate [
27]. An opposite effect (increase in stiffness with increasing MWCNT content,
Figure 4) is observed in this work. For hydrogels proposed in this work, the role of acrylamide blocks can improve resilience by additional alginate-acrylamide crosslinks (
Figure 1), as well as the weak interactions (possibly hydrogen bringing) between alginate and acrylamide blocks with oxygen-containing functionalities present on the MWCNT surface. This suggests the participation of MWCNT strands within the polymer network.
As indicated in
Figure 4E, there is a clear correlation between an increase in MWCNT content and an increase in tensile strength. Notably, the total MWCNT content of 36 mg mL
−1 showed both the best mechanical and electrochemical characteristics. Further testing at 48 mg mL
−1 indicated an oversaturation of the gel matrix, leading to unincorporated deposits of MWCNT on the surface after cross-linking was complete. The important consideration from this is the retention of physical properties of the hydrogel, with the addition of the MWCNT. Stretch was significantly reduced but retains enough capacity to serve practical applicability. An additional benefit is an increase in overall tensile force, indicating the contribution of MWCNT in the improvement of mechanical strength of hydrogels proposed in this work.
3.3. Impedance Analysis
For the polarizable electrode in the absence of redox reactions (non-faradaic measurements), the impedance signal will be dominated by the surface capacitance. This is true at least if the capacitance is low and the value of electrolyte solution is not too high. Given that electrodes in our study are symmetric, we can assume that the impedance between the electrodes can be modeled by electrolyte resistors and interfacial capacitance (
Figure 5A). The impedance spectra of dry MWCNTs showed pure resistive behavior as represented by an absolute resistance that is independent of the frequency shown in the Bode diagram (red line in
Figure 5B) and the phase shift remains at 0° in the whole frequency range (
Figure 5C).
There are three distinct regions observed in the Bode plots for pure hydrogel (black spectrum). At the highest frequencies (between 10
5 and 10
4 Hz), resistive behavior is observed where the phase shift passes through 0° and the impedance magnitude is a horizontal line (because resistive impedance is not frequency-dependent). This represents the bulk resistance of the gel/electrolyte, which is used to calculate the hydrogel conductivities. In a broad range of frequency (800–1 Hz), capacitive behavior is observed as indicated by the negative |Z| slope (
Figure 5B) and the phase angle approaching −90° (
Figure 5C), which is due to double-layer capacitance formed at the interface of the electrolyte and FTO plates, and is typically observed in this frequency range. From the narrow range (400–800 Hz), diffusion behavior is observed as the phase angle approaches −45° (and the slope of impedance magnitude has changed). This represents the diffusion of ions through the finite thickness of the hydrogel. Considering all these components, the hydrogel EIS spectra can be fitted using an equivalent circuit shown in
Figure 5A as an insert. Hydrogels containing 10, 20, and 40 mg of MWCNTs exhibited almost identical impedance magnitude over all frequencies when compared to pure hydrogel. Their slightly decreased conductivity is due to incorporated MWCNT, which has otherwise higher resistivity (80.7 Ohms, red spectrum) than pure electrolyte. When comparing pure electrolyte with the hydrogel containing 80 mg of MWCNTs, the most significant differences occurred in the mid and low frequencies, where interfacial and bulk ion diffusion is predominant.
The weakly resolved semicircles in impedance and admittance spectra (blue and green spectra,
Figure 5A) indicate multiple circuit elements. Clearly, at higher MWCNT content, the two conductivity components can be separated. The one at higher frequencies is related to the more conducive hydrogel electrolyte and the second semicircle (at the middle range of frequencies) represents an electronic conductivity of the MWCNT fraction. This component is more clearly manifested in samples with 360 mg of MWCNT, with the resistance of MWCNT fraction that is very close to that of dry MWCNT (
Figure 5, red). Yet, the total conductivity of the material is very similar to pure hydrogel over the broad range of MWCNT content. A significant change can be observed at the lowest frequency in both Bode diagrams. The magnitude of impedance decreases, and the phase angle changes from capacitive to diffusive (approaching −45°) for all hydrogels containing MWCNT. The Warburg is associated with a semi solid-state diffusion of ions in hydrogel. The Warburg coefficient representing transition time (
τ, s) reflects the diffusion through the hydrogel is inversely related to the diffusion coefficient, according to Equation (1):
where
L (m) is an effective diffusion length and
D (m
2 s
−1) is a diffusion coefficient. The transition time (
τ) is estimated based on fitting of an electrical equivalent circuit to the impedance spectra and obtaining Warburg resistor defined as:
where
Z is impedance,
ω is frequency,
R represents resistance, and
P is a constant (0 <
P < 1).
An effective diffusion length (
L, m) is calculated using Equation (1) and the results are shown in
Table 1. In general, the transition time
τ, estimated from the fitting of the Warburg element, decreases with an increasing MWCNT content in the hydrogel. This increase for the electrode containing 360 mg of MWCNT is within two orders of magnitude of that of pure hydrogel. Consequently, an effective diffusion length for ion transport,
L, decreases with increasing MWCNT amount. The trend in both, the time constant and the diffusion length, indicates that mass transport within the MWCNT-embedded hydrogels is improved as compared to the pure hydrogel. This can be rationalized as a positive effect of an increase of surface area between the electronic conductor (MWCNT) and the hydrogel electrolyte, resulting in the expansion of the double-layer interface within the material and the current collectors.
When the concentration gradient vanishes at the hydrogel/current collector interface, the Warburg impedance transforms into a capacitive behavior for pure hydrogel (black spectrum,
Figure 5A) and for all samples with an MWCNT content lower than 80 mg (green spectrum,
Figure 5A). This is demonstrated as an almost constant phase angle reaching a steady value of 80° for pure hydrogel. The low-frequency region is noticeably affected by the amount of MWCNT embedded in hydrogel. This is manifested by a change from capacitive behavior observed for pure hydrogel electrolyte to a more diffusive behavior for the MWCNT-hydrogel films. An additional diffusion process may occur at the MWCNT-hydrogel interface together with the surface diffusion of the adsorbed ions at the material/current collector. With increasing MWCNT content, this effect is stronger. Consequently, the low-frequency region of the impedance spectra for the film containing 360 mg of MWCNT shows an infinitive diffusion due to a large MWCNT-hydrogel interface across the material.
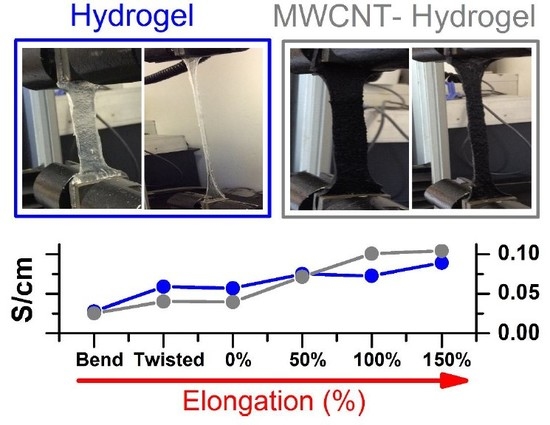
Figure 6 shows the electrochemical set-up for testing conductivity upon elongation, bending, and twisting (A), and a cross-sectional photo of the MWCNT-hydrogel (2.79 wt.% of carbon), (B) together with a total conductivity of hydrogel electrodes and electrolytes normalized to the thickness, length, and width of the film upon deformation (C), and without normalization (D). Overall, the pure hydrogel electrolyte shows slightly higher specific conductivity as compared to the MWCNT-embedded films in steady state (
Figure 6A). This decrease in total conductivity for the MWCNT-containing materials is because the resistance of pure MWCNTs is slightly higher than the hydrogel, as manifested in
Figure 5A (red spectrum of bare MWCNT). However, the hydrogel with the highest MWCNT content has higher conductivity than the bare MWCNT. From the fitting of the admittance spectra, the conductivity of ionic fraction (hydrogel) and an electronic component (MWCNT) could be resolved and is included in
Table 1. The ionic component (conductivity of pure hydrogel) is higher than the electronic one and remains constant regardless of the MWCNT content (5.2 × 10
−2 S cm
−1). The electronic counterpart evolves around values obtained for the bare MWCNT. A minor increase with increasing MWCNT content results in mixed conductivity that is close to that of pure electrolyte for the film containing 360 mg of MWCNT. This demonstrates that the hydrogel does not suppress MWCNT conductivity. The hydrogel matrix shows conductivity similar to liquid electrolyte and the MWCNT response is similar to what is typically observed in the liquid electrolyte with a similar KCl concentration [
31]. All films showed no influence of twisting at about 180 degrees on the specific conductivity (
Figure 6C,D). Furthermore, impedance was recorded during bending, and demonstrated a weak decrease when normalized to new geometries (
Figure 6C), or no difference without normalization (
Figure 6D). Interestingly, the elongation from original shape (0% elongation corresponds to as-prepared film) causes an increase in specific conductivity for all hydrogels since their thickness decreased significantly (although they were stretched to more than 150% of their original length;
Figure 7A,B shows an evolution of admittance spectra upon elongation for pure hydrogels and with 360 mg of MWCNT). All electrodes demonstrated excellent reproducibility of electrical characteristics upon multiple stretching and deformation during repetitions of the same test (unless breaking was anticipated).
Yet, hydrogels with higher MWCNT content (240 and 360 mg,
Figure 6C) showed improved total conductivity upon elongation when compared to pure hydrogel. This indicates that MWCNT not only generates electronic conductivity, but also enhances the total conductivity of the hydrogel, especially when subjected to mechanical deformations. This can be rationalized as improved tensile strength and rigidness of the MWCNT-embedded films. With that, some indication is given that weak chemical interactions between the MWCNT surface (enriched with oxygen-containing functionalities, i.e., carboxylic, phenolic, hydroxyl groups) and the acrylamide are possible, and result in improved mechanical and electrical characteristics of the combined materials. These molecular interactions can be also confirmed by the shift of onset peaks of hydrogel in the presence of MWCNT in the TGA spectrum (
Figure 2). In addition, optical observations revealed a uniform distribution of MWCNT within the hydrogel, even at high MWCNT content (
Figure 6B). The electrode shows good homogeneity and carbon dispersion across the film. This could be rationalized as both electrostatic and chemical effects i.e., related to the negative charge of MWCNT surface and alginate, and/or weak interaction between carboxyl groups present on the MWCNT surface with hydroxyl functionalities of alginate, resulting in chemical bond formation or possible electrostatic interaction. Thus, alginate not only participates in an ionic cross-linking of the base hydrogel matrix, but also modifies the MWCNT surface resulting in its better dispersion in aqueous solution, keeping in mind that these interactions are rather weak (hydrogen bridging or Van der Waal forces), unless esterification between these components is considered. At this point of the study, we can only speculate on the nature of these interactions since the FTIR did not show a significant difference between pure hydrogel and MWCNT-containing samples (
Figure 4).
3.4. Electrochemical Stability
An accelerated charge-discharge led to degradation of the hydrogel at the point of contact of materials with the FTO current collector (manifested by slightly darker color at the hydrogel/FTO contact). Furthermore, TGA analysis of as-prepared and degraded hydrogels showed no differences in the thermograms, leading to conclusions that the increase in impedance (observed only at lowest frequencies) are related to the passivation of the FTO when exposed to corrosive components such as 1 M KCl for extended periods of time. Both the thermal and spectroscopic analysis have revealed that there is no noticeable chemical change within the material after long-term charging at 0.5 mA.
The total conductivity of hydrogel decreases only slightly and within the same order of magnitude as demonstrated on the admittance spectra in
Figure 7C,D. Since the bare MWCNTs did not show any changes when subjected to charging at various current loads, we concluded that the decrease in conductivity is primarily due to the passivation of the contacts. The analysis of the Warburg element did not show any changes in time constant, or if any, the values laid within the calculation error. Yet, the decrease in admittance is slightly smaller for pure hydrogel as compared to the MWCNT-containing electrodes when tested in the long-term charge-discharge. This indicates slightly faster degradation due to the presence of MWCNT. Since the MWCNT enhances electrical contact between hydrogels, current collectors, and within materials, the degradation due to charging is accelerated.
To further estimate the practical potential windows for hydrogels, samples were polarized at various regimes and corresponding cyclic voltammograms are presented in
Figure 8. In general, all hydrogels are stable within −0.95 and +0.95 V regardless of the MWCNT content, with a slightly improved potential stability for samples containing 360 mg of MWCNT. The materials are pronounced unstable above ±1.0 V due to water evolution as revealed by strong redox signals in CVs in
Figure 8. An important observation was made on the current magnitude recorded for pure (
Figure 8B, insert) and MWCNT-embedded hydrogel (
Figure 8A). Due to the high content of MWCNT that are well-known to be double-layer capacitors, the current observed for combined hydrogel is two orders of magnitude higher when compared to pure hydrogel. Both the current and the shape of the CV spectrum indicate the strong capacitive nature of the latter, making them a potentially good candidate as an electrode material for flexible capacitor devices.
Cyclic voltammetry (
Figure 9A) was utilized to define the area-specific interfacial capacitance of the material (
Cs, F cm
−2), calculated through Equation (3):
where
ACV is the area bound by the CV (0.00284 AV),
a is the electrode surface area (12.0 mm × 12.0 mm), Δ
V is the potential window (1.6 V), and
ν is the scan rate (0.2 V s
−1). For the MWCNT-hydrogel at 3.69 wt.% of carbon, the area-specific interfacial capacitance was 27 mF cm
−2. This interfacial double-layer capacitance of the carbon is within a range of the thin-film solid-state carbon electrodes (11 mF cm
−2; [
35]). The impact of carbon content on a magnitude of charging current has been noticed only at higher MWCNT concentration (higher than 240 mg of MWCNT, that is 1.88 wt.%;
Figure 9A). This leads to the conclusion that the minimum carbon content of 2.0 wt.% is required in order to create flexible electrodes with desired electrical characteristics.
Figure 9B demonstrates a typical increase of current with increasing potential scan rate (for the electrode with 2.79 wt.% of MWCNT), showing the ability of this electrode to pass more charge. The bulk capacitance of these electrodes is expected to be much higher and will be tested in a three-electrode electrochemical cell in a future study. An increase in charging current for the carbon-containing hydrogel in comparison to the pure hydrogel electrolyte shown in
Figure 9A (black scan) indicates the viability of this conducting platform and its possible implementation in stretchable electronics.