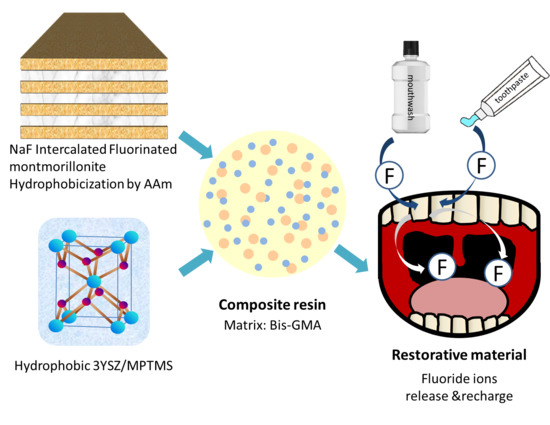
Fluorinated Montmorillonite and 3YSZ as the Inorganic Fillers in Fluoride-Releasing and Rechargeable Dental Composition Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.1.1. Preparation of the NaF-Intercalated Fluorinated Montmorillonite (FMMT/AAm-NaF) Filler
2.1.2. 3YSZ Silane Treatment with MPTMS
2.1.3. Preparation of the Composite Resin
2.2. Materials Analysis and Characterization: XRD, FTIR, TGA, and Particle Size
2.3. Mechanical Analysis: Curing Depth, Hardness, Diametral Tensile Strength, Flexural Strength, and Wear Resistance
2.4. Oral Environment Simulation
2.4.1. Fluoride Release Measurement
2.4.2. Fluoride Recharge Measurement
2.5. Biocompatibility
WST-1 Assay
2.6. Statistical Analysis
3. Results
3.1. Material Characterization: XRD, FTIR, TGA, and Particle Size
3.1.1. XRD
3.1.2. FTIR
3.1.3. TGA
3.1.4. Particle Size
3.2. Mechanical Analysis: Curing Depth, Hardness, Diametral Tensile Strength, Flexural Strength, and Wear Resistance
3.2.1. Curing Depth
3.2.2. Hardness, Diametral Tensile Strength, Flexural Strength, and Wear Resistance
3.3. Oral Environment Simulation.
3.3.1. Fluoride Release Measurement
3.3.2. Fluoride Recharge Measurement
3.4. Biocompatibility (WST-1 Assay)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, P.E.; Lennon, M.A. Effective use of fluorides for the prevention of dental caries in the 21st century: The WHO approach. Community Dent. Oral Epidemiol. 2004, 32, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Dixit, L.P.; Shakya, A.; Shrestha, M.; Shrestha, A. Dental caries prevalence, oral health knowledge and practice among indigenous Chepang school children of Nepal. BMC Oral Health 2013, 13, 20. [Google Scholar]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan use in dentistry: A systematic review of recent clinical studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef] [Green Version]
- García-Godoy, F.; Hicks, M.J. Maintaining the integrity of the enamel surface: The role of dental biofilm, saliva and preventive agents in enamel demineralization and remineralization. J. Am. Dent. Assoc. 2008, 139, 25S–34S. [Google Scholar]
- Marsh, P. Sugar, fluoride, pH and microbial homeostasis in dental plaque. Proc. Finn. Dent. Soc. 1991, 87, 515–525. [Google Scholar]
- Pollick, H. The Role of Fluoride in the Prevention of Tooth Decay. Pediatr. Clin. N. Am. 2018, 65, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. Prevention and reversal of dental caries: Role of low level fluoride. Community Dent. Oral Epidemiol. 1999, 27, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Eichmiller, F.; Marjenhoff, W. Fluoride-releasing dental restorative materials. Oper. Dent. 1998, 23, 218–228. [Google Scholar]
- Zafar, M.S.; Ahmed, N. Therapeutic roles of fluoride released from restorative dental materials. Fluoride 2015, 48, 184–194. [Google Scholar]
- Sakaguchi, R.L.; Powers, J.M. Craig’s Restorative Dental Materials-E-Book, 13th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Caughman, W.F.; Kovarik, R.; Rueggeberg, F.; Snipes, W. The bond strength of Panavia Ex to air-abraded amalgam. Int. J. Prosthodont. 1991, 4, 276–281. [Google Scholar]
- Suh, B.I. All-Bond—Fourth Generation Dentin Bonding System. J. Esthet. Dent. 1991, 3, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ucar, Y.; Akova, T.; Aysan, I. Mechanical properties of polyamide versus different PMMA denture base materials. J. Prosthodont. 2012, 21, 173–176. [Google Scholar] [CrossRef]
- Forsten, L. Resin-modified glass ionomer cements: Fluoride release and uptake. Acta Odontol. Scand. 1995, 53, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Rix, D.; Foley, T.F.; Banting, D.; Mamandras, A. A comparison of fluoride release by resin-modified GIC and polyacid-modified composite resin. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 398–405. [Google Scholar] [CrossRef]
- Koch, G.; Hatibović-Kofman, S. Glass ionomer cements as a fluoride release system in vivo. Swed. Dent. J. 1990, 14, 267–273. [Google Scholar] [PubMed]
- Momoi, Y.; McCabe, J. Fluoride release from light-activated glass ionomer restorative cements. Dent. Mater. 1993, 9, 151–154. [Google Scholar] [CrossRef]
- Davallo, M.; Pasdar, H.; Mohseni, M. Mechanical properties of unsaturated polyester resin. Int. J. ChemTech Res. 2010, 2, 2113–2117. [Google Scholar]
- Magni, E.; Ferrari, M.; Hickel, R.; Ilie, N. Evaluation of the mechanical properties of dental adhesives and glass-ionomer cements. Clin. Oral Investig. 2010, 14, 79–87. [Google Scholar] [CrossRef]
- Kanerva, L.; Estlander, T.; Jolanki, R. Occupational skin allergy in the dental profession. Dermatol. Clin. 1994, 12, 517–532. [Google Scholar] [CrossRef]
- Glenn, J.F. Comments on Dr. Bowen’s presentation. J. Dent. Res. 1979, 58, 1504–1506. [Google Scholar]
- Khatri, C.A.; Stansbury, J.W.; Schultheisz, C.R.; Antonucci, J.M. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent. Mater. 2003, 19, 584–588. [Google Scholar] [CrossRef]
- Glasspoole, E.; Erickson, R.; Davidson, C. A fluoride-releasing composite for dental applications. Dent. Mater. 2001, 17, 127–133. [Google Scholar] [CrossRef]
- Xu, X.; Burgess, J.O. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 2003, 24, 2451–2461. [Google Scholar] [CrossRef]
- Li, K.-Y.; Tsai, C.-C.; Fang, C.-H.; Wang, Y.-L.; Lin, F.-H.; Lin, C.-P. Fluorinated Montmorillonite Composite Resin as a Dental Pit and Fissure Sealant. Polymers 2019, 11, E1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, F.; Phillips, R.W. A classification and evaluation of composite resin systems. J. Prosthet. Dent. 1983, 50, 480–488. [Google Scholar] [CrossRef]
- Ghatee, M.; Shariat, M.; Irvine, J. Investigation of electrical and mechanical properties of 3YSZ/8YSZ composite electrolytes. Solid State Ion. 2009, 180, 57–62. [Google Scholar] [CrossRef]
- Theng, K.Y.; Muchtar, A.; Yahaya, N.; Ghazali, M.J. Development of translucent zirconia for dental crown applications. Asian J. Sci. Res. 2015, 8, 342–350. [Google Scholar]
- Pradhan, M.; Kapur, P. Effect of powder dispersion on sintering behavior and mechanical properties of nanostructured 3YSZ ceramics. Cera. Int. 2012, 38, 2835–2843. [Google Scholar] [CrossRef]
- Tredici, I.G.; Sebastiani, M.; Massimi, F.; Bemporad, E.; Resmini, A.; Merlati, G.; Anselmi-Tamburini, U. Low temperature degradation resistant nanostructured yttria-stabilized zirconia for dental applications. Cera. Int. 2016, 42, 8190–8197. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Dickens, S.H.; Fowler, B.O.; Xu, H.H.; McDonough, W.G. Chemistry of silanes: Interfaces in dental polymers and composites. J. Res. Nat. Inst. Stand. Technol. 2005, 110, 541. [Google Scholar] [CrossRef]
- Flury, S.; Hayoz, S.; Peutzfeldt, A.; Hüsler, J.; Lussi, A. Depth of cure of resin composites: Is the ISO 4049 method suitable for bulk fill materials? Dent. Mater. 2012, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Casselli, D.S.M.; Worschech, C.C.; Paulillo, L.A.; Dias, C.T. Diametral tensile strength of composite resins submitted to different activation techniques. Braz. Oral Res. 2006, 20, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Paes, P.N.G.; Miranda, M.S.; Sampaio-Filho, H.R.; Correr-Sobrinho, L. Influence of activation mode, fatigue, and ceramic interposition on resin cements’ diametral tensile strength. Braz. Oral Res. 2019, 33, e083. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Mehdawi, I.M.; Sakai, T.; Abe, T.; Inoue, S.; Imazato, S. In vitro/in silico investigation of failure criteria to predict flexural strength of composite resins. Dent. Mater. J. 2018, 37, 152–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Song, S.-Y.; Lee, K.-S.; Park, J.-H.; Ryu, J.-J.; Lee, J.-Y. Effects of relining materials on the flexural strength of relined thermoplastic denture base resins. J. Adv. Prosthodont. 2018, 10, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-L.; Lee, B.-S.; Chang, K.-C.; Chiu, H.-C.; Lin, F.-H.; Lin, C.-P. Characterization, fluoride release and recharge properties of polymer–kaolinite nanocomposite resins. J. Comp. Sci. Techn. 2007, 67, 3409–3416. [Google Scholar] [CrossRef]
- Wallin, R.F.; Arscott, E. A practical guide to ISO 10993-5: Cytotoxicity. MDDI 1998, 20, 96–98. [Google Scholar]
- ISO. 10993-12: 2008–Biological evaluation of medical devices–Part 12: Sample preparation and reference materials (ISO 10993-12: 2007). German version: DIN EN ISO. 2008. 10993-10912. Available online: https://www.iso.org/standard/53468.html (accessed on 16 January 2020).
- Bringley, J.F.; Liebert, N.B. Controlled chemical and drug delivery via the internal and external surfaces of layered compounds. J. Dispers. Sci. Technol. 2003, 24, 589–605. [Google Scholar] [CrossRef]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef]
- Murugan, R.; Mohan, S.; Bigotto, A. FTIR and polarised Raman spectra of acrylamide and polyacrylamide. J. Korean Phys. Soc. 1998, 32, 505–512. [Google Scholar]
- Okamoto, M.; Morita, S.; Kotaka, T. Dispersed structure and ionic conductivity of smectic clay/polymer nanocomposites. Polymer 2001, 42, 2685–2688. [Google Scholar] [CrossRef]
- Okamoto, M.; Morita, S.; Kim, Y.; Kotaka, T.; Tateyama, H. Dispersed structure change of smectic clay/poly (methyl methacrylate) nanocomposites by copolymerization with polar comonomers. Polymer 2001, 42, 1201–1206. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Fowler, B.O.; Dickens, S.H.; Richards, N.D. Novel Dental Resins from Trialkoxysilanes and Dental Monomers by in Situ Formation of Oligomeric Silyl Ethers and Silsesquioxines. In Polymer Preprints; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Pilo, R.; Dimitriadi, M.; Palaghia, A.; Eliades, G. Effect of tribochemical treatments and silane reactivity on resin bonding to zirconia. Dent. Mater. 2018, 34, 306–316. [Google Scholar] [CrossRef]
- Park, O.-H.; Jung, J.-I.; Bae, B.-S. Photoinduced condensation of sol-gel hybrid glass films doped with benzildimethylketal. J. Mater. Res. 2001, 16, 2143–2148. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.K.; Platt, J.; Borges, G.; Chu, T.G.; Katsilieri, I. Depth of cure of dental resin composites: ISO 4049 depth and microhardness of types of materials and shades. Oper. Dent. 2008, 33, 408–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuyama, K.; Murata, Y.; Pereira, P.; Miguez, P.; Komatsu, H.; Sano, H. Fluoride release and uptake by various dental materials after fluoride application. Am. J. Dent. 2006, 19, 123–127. [Google Scholar] [PubMed]
- Rosa, R.S.; Balbinot, C.E.A.; Blando, E.; Mota, E.G.; Oshima, H.M.S.; Hirakata, L.; Pires, L.A.G.; Hübler, R. Evaluation of mechanical properties on three nanofilled composites. Stomatologija 2012, 14, 126–130. [Google Scholar]
- Kinomoto, Y.; Torii, M.; Takeshige, F.; Ebisu, S. Comparison of polymerization contraction stresses between self-and light-curing composites. J. Dent. 1999, 27, 383–389. [Google Scholar] [CrossRef]
- Rao, B.S.R.; Moosani, G.K.R.; Shanmugaraj, M.; Kannapan, B.; Shankar, B.S.; Ismail, P.M.S. Fluoride release and uptake of five dental restoratives from mouthwashes and dentifrices. J. Int. Oral Health 2015, 7, 1–5. [Google Scholar]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.-Y.; Tsai, C.-C.; Lin, T.-C.; Wang, Y.-L.; Lin, F.-H.; Lin, C.-P. Fluorinated Montmorillonite and 3YSZ as the Inorganic Fillers in Fluoride-Releasing and Rechargeable Dental Composition Resin. Polymers 2020, 12, 223. https://doi.org/10.3390/polym12010223
Li K-Y, Tsai C-C, Lin T-C, Wang Y-L, Lin F-H, Lin C-P. Fluorinated Montmorillonite and 3YSZ as the Inorganic Fillers in Fluoride-Releasing and Rechargeable Dental Composition Resin. Polymers. 2020; 12(1):223. https://doi.org/10.3390/polym12010223
Chicago/Turabian StyleLi, Keng-Yuan, Cheng-Chia Tsai, Tzu-Chieh Lin, Yin-Lin Wang, Feng-Huei Lin, and Chun-Pin Lin. 2020. "Fluorinated Montmorillonite and 3YSZ as the Inorganic Fillers in Fluoride-Releasing and Rechargeable Dental Composition Resin" Polymers 12, no. 1: 223. https://doi.org/10.3390/polym12010223
APA StyleLi, K. -Y., Tsai, C. -C., Lin, T. -C., Wang, Y. -L., Lin, F. -H., & Lin, C. -P. (2020). Fluorinated Montmorillonite and 3YSZ as the Inorganic Fillers in Fluoride-Releasing and Rechargeable Dental Composition Resin. Polymers, 12(1), 223. https://doi.org/10.3390/polym12010223