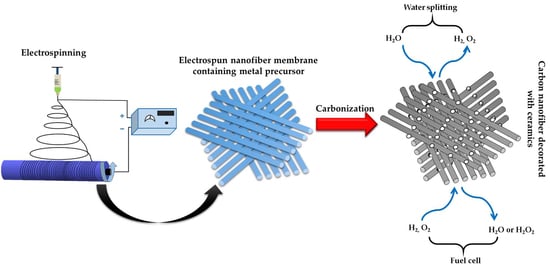
Electrospun CNF Supported Ceramics as Electrochemical Catalysts for Water Splitting and Fuel Cell: A Review
Abstract
:1. Introduction
2. Brief Background of Electrocatalysis: HER, OER, HOR and ORR
2.1. Hydrogen Evolution Reaction and Oxygen Evolution Reaction
2.2. Hydrogen Oxidation Reaction and Oxygen Reduction Reaction
3. Brief Background of Electrospinning
4. CNF-Supported Metal Oxides/Carbides/Phosphides/Sulfides as Electrocatalysts
4.1. CNF Supported Metal Oxides
4.2. CNF Supported Metal Carbides
4.3. CNF Supported Metal Phosphides
4.4. CNF-Supported Metal Sulfides
5. Conclusions
Funding
Conflicts of Interest
References
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- Santos, E.; Schmickler, W. (Eds.) Catalysis in Electrochemistry: From Fundamental Aspects to Strategies for Fuel Cell Development; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 7. [Google Scholar]
- Vion, H.C. Improved Method of Utilizing Atmospheric Electricity. U.S. Patent No. 28793A, 19 June 1860. [Google Scholar]
- Kumar, B.; Asadi, M.; Pisasale, D.; Sinha-Ray, S.; Rosen, B.A.; Haasch, R.; Abiade, J.; Yarin, A.L.; Salehi-Khojin, A. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat. Commun. 2013, 4, 2819. [Google Scholar] [CrossRef]
- Chen, S.; Hou, H.; Harnisch, F.; Patil, S.A.; Carmona-Martinez, A.A.; Agarwal, S.; Zhang, Y.; Sinha-Ray, S.; Yarin, A.L.; Greiner, A.; et al. Electrospun and solution blown three-dimensional carbon fiber nonwovens for application as electrodes in microbial fuel cells. Energy Environ. Sci. 2011, 4, 1417–1421. [Google Scholar] [CrossRef]
- Borup, R.L.; Davey, J.R.; Garzon, F.H.; Wood, D.L.; Inbody, M.A. PEM fuel cell electrocatalyst durability measurements. J. Power Sources 2006, 163, 76–81. [Google Scholar] [CrossRef]
- McCrory, C.C.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen electrocatalysts in metal–air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iea.org/geco/electricity/ (accessed on 1 December 2019).
- EG&G Technical Services. Fuel Cell Handbook, 7th ed.; Department of Energy Inc.: Washington, DC, USA, 2004. Available online: https://www.osti.gov/servlets/purl/834188 (accessed on 14 December 2019).
- Badwal, S.P.S.; Foger, K. Solid oxide electrolyte fuel cell review. Ceram. Int. 1996, 22, 257–265. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P. Guest editorial: Chemistry and physics of nanowires. Adv. Mater. 2003, 15, 351–352. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific Publishing Co Pte Ltd.: Singapore, 2011; pp. 148–159. [Google Scholar]
- Sides, C.R.; Martin, C.R. Nanostructured electrodes and the low-temperature performance of li-ion batteries. Adv. Mater. 2005, 17, 125–128. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wu, Y. Mesoporous Co3O4 nanowire arrays for lithium ion batteries with high capacity and rate capability. Nano Lett. 2008, 8, 265–270. [Google Scholar] [CrossRef]
- Cui, L.F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-amorphous core−shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 2008, 9, 491–495. [Google Scholar] [CrossRef]
- Liu, B.; Aydil, E.S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Chang, K.H.; Lin, M.C.; Wu, Y.T. Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Lett. 2006, 6, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
- Kakoria, A.; Devi, B.; Anand, A.; Halder, A.; Koner, R.R.; Sinha-Ray, S. Gallium oxide nanofibers for hydrogen evolution and oxygen reduction. ACS Appl. Nano Mater. 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Tan, H.; Cai, R.; Shi, W.; Li, C.; Mhaisalkar, S.G.; Srinivasan, M.; Ramakrishna, S.; Yan, Q. MS2 (M = Co and Ni) hollow spheres with tunable interiors for high-performance supercapacitors and photovoltaics. Adv. Funct. Mater. 2014, 24, 2155–2162. [Google Scholar] [CrossRef]
- Yu, H.; Guo, J.; Zhu, S.; Li, Y.; Zhang, Q.; Zhu, M. Preparation of continuous alumina nanofibers via electrospinning of PAN/DMF solution. Mater. Lett. 2012, 74, 247–249. [Google Scholar] [CrossRef]
- Dong, Z.; Kennedy, S.J.; Wu, Y. Electrospinning materials for energy-related applications and devices. J. Power Sources 2011, 196, 4886–4904. [Google Scholar] [CrossRef]
- Sahay, R.; Kumar, P.S.; Sridhar, R.; Sundaramurthy, J.; Venugopal, J.; Mhaisalkar, S.G.; Ramakrishna, S. Electrospun composite nanofibers and their multifaceted applications. J. Mater. Chem. 2012, 22, 12953–12971. [Google Scholar] [CrossRef]
- Xu, J.J.; Xu, D.; Wang, Z.L.; Wang, H.G.; Zhang, L.L.; Zhang, X.B. Synthesis of perovskite-based porous La0.75Sr0.25MnO3 nanotubes as a highly efficient electrocatalyst for rechargeable lithium–oxygen batteries. Angew. Chem. Int. Ed. 2013, 52, 3887–3890. [Google Scholar] [CrossRef]
- Cai, Y.; Ke, H.; Dong, J.; Wei, Q.; Lin, J.; Zhao, Y.; Song, L.; Hu, Y.; Huang, F.; Gao, W.; et al. Effects of nano-SiO2 on morphology, thermal energy storage, thermal stability, and combustion properties of electrospun lauric acid/PET ultrafine composite fibers as form-stable phase change materials. Appl. Energy 2011, 88, 2106–2112. [Google Scholar] [CrossRef]
- Aravindan, V.; Sundaramurthy, J.; Jain, A.; Kumar, P.S.; Ling, W.C.; Ramakrishna, S.; Srinivasan, M.P.; Madhavi, S. Unveiling TiNb2O7 as an insertion anode for lithium ion capacitors with high energy and power density. ChemSusChem 2014, 7, 1858–1863. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, K.; Niu, C.; Zhang, L.; Cai, Z.; Han, C.; He, L.; Shen, T.; Yan, M.; Qu, L.; et al. Heterogeneous branched core–shell SnO2–PANI nanorod arrays with mechanical integrity and three dimensional electron transport for lithium batteries. Nano Energy 2014, 8, 196–204. [Google Scholar] [CrossRef]
- Ren, W.; Zheng, Z.; Luo, Y.; Chen, W.; Niu, C.; Zhao, K.; Yan, M.; Zhang, L.; Meng, J.; Mai, L. An electrospun hierarchical LiV3O8 nanowire-in-network for high-rate and long-life lithium batteries. J. Mater. Chem. A 2015, 3, 19850–19856. [Google Scholar] [CrossRef]
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of semiconducting oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Xu, B.; Gu, Y.; Xiong, Z.; Sun, J.; Zhao, X.S. Graphene-based electrodes for electrochemical energy storage. Energy Environ. Sci. 2013, 6, 1388–1414. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, F.; Tarascon, J.M.; Kim, J.K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Wei, Y. One-dimensional composite nanomaterials: Synthesis by electrospinning and their applications. Small 2009, 5, 2349–2370. [Google Scholar] [CrossRef]
- Tripković, V.; Abild-Pedersen, F.; Studt, F.; Cerri, I.; Nagami, T.; Bligaard, T.; Rossmeisl, J. Metal oxide-supported platinum over layers as proton-exchange membrane fuel cell cathodes. ChemCatChem 2012, 4, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Liu, J.; Gu, J.; Su, L.; Cheng, L. An overview of metal oxide materials as electrocatalysts and supports for polymer electrolyte fuel cells. Energy Environ. Sci. 2014, 7, 2535–2558. [Google Scholar] [CrossRef]
- Peng, L.; Shah, S.S.A.; Wei, Z. Recent developments in metal phosphide and sulfide electrocatalysts for oxygen evolution reaction. Chin. J. Catal. 2018, 39, 1575–1593. [Google Scholar] [CrossRef]
- Li, W.; Gao, X.; Wang, X.; Xiong, D.; Huang, P.P.; Song, W.G.; Bao, X.; Liu, L. From water reduction to oxidation: Janus Co-Ni-P nanowires as high-efficiency and ultrastable electrocatalysts for over 3000 h water splitting. J. Power Sources 2016, 330, 156–166. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, J.; Shi, T.; Zhou, X.; Bai, X.; Huang, J.Y. Nitrogen-doped ultrathin carbon nanofibers derived from electrospinning: Large-scale production, unique structure, and application as electrocatalysts for oxygen reduction. J. Power Sources 2011, 196, 9862–9867. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Kamble, A.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Electrospinning: A versatile technique for making of 1D growth of nanostructured nanofibers and its applications: An experimental approach. Appl. Surf. Sci. 2017, 423, 641–674. [Google Scholar] [CrossRef]
- Ramaseshan, R.; Sundarrajan, S.; Jose, R.; Ramakrishna, S. Nanostructured ceramics by electrospinning. J. Appl. Phys. 2007, 102, 7. [Google Scholar] [CrossRef]
- Sigmund, W.; Yuh, J.; Park, H.; Maneeratana, V.; Pyrgiotakis, G.; Daga, A.; Taylor, J.; Nino, J.C. Processing and structure relationships in electrospinning of ceramic fiber systems. J. Am. Ceram. Soc. 2006, 89, 395–407. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—A review. J. Mater. Process. Technol. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Al-Ajrah, S.; Lafdi, K.; Liu, Y.; Le Coustumer, P. Fabrication of ceramic nanofibers using polydimethylsiloxane and polyacrylonitrile polymer blends. J. Appl. Polym. Sci. 2018, 135, 45967. [Google Scholar] [CrossRef]
- Panda, P.K. Ceramic nanofibers by electrospinning technique—A review. Trans. Indian Ceramic Soc. 2007, 66, 65–76. [Google Scholar] [CrossRef]
- Sawicka, K.M.; Gouma, P. Electrospun composite nanofibers for functional applications. J. Nanoparticle Res. 2006, 8, 769–781. [Google Scholar] [CrossRef]
- Bender, E.T.; Katta, P.; Lotus, A.; Park, S.J.; Chase, G.G.; Ramsier, R.D. Identification of CO2 sequestered in electrospun metal oxide nanofibers. Chem. Phys. Lett. 2006, 423, 302–305. [Google Scholar] [CrossRef]
- Fu, Z.W.; Ma, J.; Qin, Q.Z. Nanostructured LiCoO2 and LiMn2O4 fibers fabricated by a high frequency electrospinning. Solid State Ion. 2005, 176, 1635–1640. [Google Scholar] [CrossRef]
- Yang, X.; Shao, C.; Liu, Y.; Mu, R.; Guan, H. Nanofibers of CeO2 via an electrospinning technique. Thin Solid Film. 2005, 478, 228–231. [Google Scholar] [CrossRef]
- La Monaca, A.; Paolella, A.; Guerfi, A.; Rosei, F.; Zaghib, K. Electrospun ceramic nanofibers as 1D solid electrolytes for lithium batteries. Electrochem. Commun. 2019, 19, 106483. [Google Scholar] [CrossRef]
- Viswanathamurthi, P.; Bhattarai, N.; Kim, H.Y.; Khil, M.S.; Lee, D.R.; Suh, E.K. GeO2 fibers: Preparation, morphology and photoluminescence property. J. Chem. Phys. 2004, 121, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shao, C.; Guan, H.; Li, X.; Gong, J. Preparation and characterization of ZnO nanofibers by using electrospun PVA/zinc acetate composite fiber as precursor. Inorg. Chem. Commun. 2004, 7, 176–178. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Park, H.C.; Kim, C.K.; Kim, H.Y.; Lee, D.R. Nickel titanate nanofibers by electrospinning. Mater. Chem. Phys. 2004, 87, 5–9. [Google Scholar] [CrossRef]
- Viswanathamurthi, P.; Bhattarai, N.; Kim, H.Y.; Lee, D.R.; Kim, S.R.; Morris, M.A. Preparation and morphology of niobium oxide fibres by electrospinning. Chem. Phys. Lett. 2003, 374, 79–84. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Wang, Y.; Li, X.; Feng, S.; Wang, C. Facile synthesis of luminescent one-dimensional nanostructural praseodymium oxyfluoride. Mater. Sci. Eng. B 2008, 147, 69–73. [Google Scholar] [CrossRef]
- Ou, Y.; Zou, D.; Wang, F.; Cheng, J. Effect of synthesis parameters on the morphology of nanocrystal thermoelectric M (=Mg, Mn, Cu) doped Ca3Co4O9 fibers by electrospinning. Mater. Today Proc. 2018, 5, 25900–25905. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, D.; Zhao, Q.; Wang, C.; Zhang, W.; Wei, Y. Large-scale synthesis of necklace-like single-crystalline PbTiO3 nanowires. Macromol. Rapid Commun. 2006, 27, 76–80. [Google Scholar] [CrossRef]
- Dai, H.; Gong, J.; Kim, H.; Lee, D. A novel method for preparing ultra-fine alumina-borate oxide fibres via an electrospinning technique. Nanotechnology 2002, 13, 674. [Google Scholar] [CrossRef]
- Mohammad, N.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S. A review on synthesis and characterization of solid acid materials for fuel cell applications. J. Power Sources 2016, 322, 77–92. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Mao, J.; Guo, Q.; Chen, Z.; Lai, Y. Metal-Organic Framework/Graphene Composites: Preparation and Applications in Electrocatalysis and Photocatalysis: A review. J. Mater. Chem. A 2020. [Google Scholar] [CrossRef]
- Borgschulte, A.; Rector, J.H.; Dam, B.; Griessen, R.; Züttel, A. The role of niobium oxide as a surface catalyst for hydrogen absorption. J. Catal. 2005, 235, 353–358. [Google Scholar] [CrossRef]
- Sun, D.; Shen, Y.; Zhang, W.; Yu, L.; Yi, Z.; Yin, W.; Wang, D.; Huang, Y.; Wang, J.; Wang, D.; et al. A solution-phase bifunctional catalyst for lithium–oxygen batteries. J. Am. Chem. Soc. 2014, 136, 8941–8946. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Pagliaro, M.; Konstandopoulos, A.G. Solar Hydrogen: Fuel of the Future; Royal Society of Chemistry: London, UK, 2012. [Google Scholar]
- Vielstich, W.; Lamm, A.; Gasteiger, H.A. (Eds.) Handbook of Fuel Cells: Fundamentals Technology and Applications; Wiley: New York, NY, USA, 2003; Volume 2. [Google Scholar]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zheng, G. One-dimensional earth-abundant nanomaterials for water-splitting electrocatalysts. Adv. Sci. 2017, 4, 1600380. [Google Scholar] [CrossRef] [PubMed]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Koper, M.T. Theory of multiple proton–electron transfer reactions and its implications for electrocatalysis. Chem. Sci. 2013, 4, 2710–2723. [Google Scholar] [CrossRef] [Green Version]
- Mahata, A.; Nair, A.S.; Pathak, B. Recent advancements in Pt-nanostructure-based electrocatalysts for the oxygen reduction reaction. Catal. Sci. Technol. 2019, 9, 4835–4863. [Google Scholar] [CrossRef]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride nanosheet–carbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 7281–7285. [Google Scholar] [CrossRef]
- Gorlin, Y.; Jaramillo, T.F. A bifunctional nonprecious metal catalyst for oxygen reduction and water oxidation. J. Am. Chem. Soc. 2010, 132, 13612–13614. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Kamarudin, S.K. Platinum-Based Catalysts on Various Carbon Supports and Conducting Polymers for Direct Methanol Fuel Cell Applications: A Review. Nanoscale Res. Lett. 2018, 13, 410. [Google Scholar] [CrossRef] [Green Version]
- Davydova, E.S.; Mukerjee, S.; Jaouen, F.; Dekel, D.R. Electrocatalysts for hydrogen oxidation reaction in alkaline electrolytes. ACS Catal. 2018, 8, 6665–6690. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Jin, Y.; Qiao, S.Z. Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction. Small 2012, 8, 3550–3566. [Google Scholar] [CrossRef]
- Baschuk, J.J.; Li, X. Carbon monoxide poisoning of proton exchange membrane fuel cells. Int. J. Energy Res. 2001, 25, 695–713. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Sun, S. Tuning nanoparticle catalysis for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Shen, Z.; Thompson, B.E.; McHugh, M.A. Electrospinning in near-critical CO2. Macromolecules 2006, 39, 8553–8555. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Sinha-Ray, S. Spray in Polymer Processing. In Droplet and Spray Transport: Paradigms and Applications; Basu, S., Agarwal, A., Mukhopadhyay, A., Patel, C., Eds.; Springer: Singapore, 2018. [Google Scholar]
- Meesters, G.M.H.; Vercoulen, P.H.W.; Marijnissen, J.C.M.; Scarlett, B. Generation of micron-sized droplets from the Taylor cone. J. Aerosol Sci. 1992, 23, 37–49. [Google Scholar] [CrossRef]
- Yarin, A.L.; Zussman, E. Upward needleless electrospinning of multiple nanofibers. Polymer 2004, 45, 2977–2980. [Google Scholar] [CrossRef]
- Su, D.S.; Schlögl, R. Nanostructured carbon and carbon nanocomposites for electrochemical energy storage applications. ChemSusChem 2010, 3, 136–168. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Y.; Liu, Y.; Huang, Z.D.; He, Y.B.; Kim, J.K. Percolation threshold of graphene nanosheets as conductive additives in Li4 Ti5O12 anodes of Li-ion batteries. Nanoscale 2013, 5, 2100–2106. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, Q.; Zhang, Q.; Li, J. In situ growth of mesoporous SnO2 on multiwalled carbon nanotubes: A novel composite with porous-tube structure as anode for lithium batteries. Adv. Funct. Mater. 2007, 17, 2772–2778. [Google Scholar] [CrossRef]
- Shanmugapriya, S.; Zhu, P.; Yan, C.; Asiri, A.M.; Zhang, X.; Selvan, R.K. Multifunctional high-performance electrocatalytic properties of Nb2O5 incorporated carbon nanofibers as Pt support catalyst. Adv. Mater. Interfaces 2019, 6, 1900565. [Google Scholar] [CrossRef]
- Jung, K.N.; Lee, J.I.; Yoon, S.; Yeon, S.H.; Chang, W.; Shin, K.H.; Lee, J.W. Manganese oxide/carbon composite nanofibers: Electrospinning preparation and application as a bi-functional cathode for rechargeable lithium–oxygen batteries. J. Mater. Chem. 2012, 22, 21845–21848. [Google Scholar] [CrossRef]
- Yu, J.; Chen, G.; Sunarso, J.; Zhu, Y.; Ran, R.; Zhu, Z.; Zhou, W.; Shao, Z. Cobalt Oxide and Cobalt-Graphitic Carbon Core–Shell Based Catalysts with Remarkably High Oxygen Reduction Reaction Activity. Adv. Sci. 2016, 3, 1600060. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.E.; Li, F.; Lu, H.; Yan, J.; Huang, Y.; Liu, T. Nanocubic-Co3O4 coupled with nitrogen-doped carbon nanofiber network: A synergistic binder-free catalyst toward oxygen reduction reactions. Compos. Commun. 2016, 1, 15–19. [Google Scholar] [CrossRef]
- Eftekhari, A.; Mohamedi, M. Tailoring pseudocapacitive materials from a mechanistic perspective. Mater. Today Energy 2017, 6, 211–229. [Google Scholar] [CrossRef]
- Li, T.; Lv, Y.; Su, J.; Wang, Y.; Yang, Q.; Zhang, Y.; Zhou, J.; Xu, L.; Sun, D.; Tang, Y. Anchoring CoFe2O4 nanoparticles on N-doped carbon nanofibers for high-performance oxygen evolution reaction. Adv. Sci. 2017, 4, 1700226. [Google Scholar] [CrossRef]
- Busacca, C.; Zignani, S.C.; Di Blasi, A.; Di Blasi, O.; Faro, M.L.; Antonucci, V.; Aricò, A.S. Electrospun NiMn2O4 and NiCo2O4 spinel oxides supported on carbon nanofibers as electrocatalysts for the oxygen evolution reaction in an anion exchange membrane-based electrolysis cell. Int. J. Hydrog. Energy 2019, 44, 20987–20996. [Google Scholar] [CrossRef]
- Wang, H.; Lee, H.W.; Deng, Y.; Lu, Z.; Hsu, P.C.; Liu, Y.; Lin, D.; Cui, Y. Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting. Nat. Commun. 2015, 6, 7261. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Ge, X.; Goh, F.T.; Hor, T.A.; Geng, D.; Du, G.; Liu, Z.; Zhang, J.; Liu, X.; Zong, Y. Co3O4 nanoparticles decorated carbon nanofiber mat as binder-free air-cathode for high performance rechargeable zinc-air batteries. Nanoscale 2015, 7, 1830–1838. [Google Scholar] [CrossRef]
- Li, B.; Chien, S.W.; Ge, X.; Chai, J.; Goh, X.Y.; Nai, K.T.; Hor, T.A.; Liu, Z.; Zong, Y. Ni/NiOx-decorated carbon nanofibers with enhanced oxygen evolution activity for rechargeable zinc–air batteries. Mater. Chem. Front. 2017, 1, 677–682. [Google Scholar] [CrossRef]
- Chinnappan, A.; Dongxiao, J.; Jayathilaka, W.A.D.M.; Baskar, C.; Qin, X.; Ramakrishna, S. Facile synthesis of electrospun C@ NiO/Ni nanofibers as an electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2018, 43, 15217–15224. [Google Scholar] [CrossRef]
- Wu, M.; Fan, L.; Ma, R.; Zhu, J.; Gu, S.; Wang, T.; Gong, D.; Xu, Z.; Lu, B. NiO and CrO3 double surface-decorate Ni nanofibers for hydrogen evolution reduction. Mater. Lett. 2016, 182, 15–18. [Google Scholar] [CrossRef]
- Shinde, P.A.; Jun, S.C. Review on Recent Progress in the Development of Tungsten Oxide Based Electrodes for Electrochemical Energy Storage. ChemSusChem 2019, 2, 11–38. [Google Scholar] [CrossRef] [Green Version]
- Rees, E.J.; Brady, C.D.A.; Burstein, G.T. Solid-state synthesis of tungsten carbide from tungsten oxide and carbon, and its catalysis by nickel. Mater. Lett. 2008, 62, 1–3. [Google Scholar] [CrossRef]
- Chen, J.; Yu, D.; Liao, W.; Zheng, M.; Xiao, L.; Zhu, H.; Zhang, M.; Du, M.; Yao, J. WO3–x Nanoplates Grown on Carbon Nanofibers for an Efficient Electrocatalytic Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 18132–18139. [Google Scholar] [CrossRef]
- Tovini, M.F.; Patil, B.; Koz, C.; Uyar, T.; Yılmaz, E. Nanohybrid structured RuO2/Mn2O3/CNF as a catalyst for Na–O2 batteries. Nanotechnology 2018, 29, 475401. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Liu, X.; Yue, X.; Jia, J.; Guo, S. Bamboo-like carbon nanotube/Fe3C nanoparticle hybrids and their highly efficient catalysis for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 1436–1439. [Google Scholar] [CrossRef]
- Jeong, B.; Shin, D.; Choun, M.; Maurya, S.; Baik, J.; Mun, B.S.; Moon, S.H.; Su, D.; Lee, J. Nitrogen-deficient ORR active sites formation by iron-assisted water vapor activation of electrospun carbon nanofibers. J. Phys. Chem. C 2016, 120, 7705–7714. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef]
- An, G.H.; Lee, Y.G.; Ahn, H.J. Multi-active sites of iron carbide nanoparticles on nitrogen@cobalt-doped carbon for a highly efficient oxygen reduction reaction. J. Alloys Compd. 2018, 746, 177–184. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Guo, X.; Fan, H.; Wu, W.; Liu, H.; Wang, G. Fe3C@ nitrogen doped CNT arrays aligned on nitrogen functionalized carbon nanofibers as highly efficient catalysts for the oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 19672–19679. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Wang, H.; Wang, C.; Pinna, N.; Lu, X. Ni strongly coupled with mo2c encapsulated in nitrogen-doped carbon nanofibers as robust bifunctional catalyst for overall water splitting. Adv. Energy Mater. 2019, 9, 1803185. [Google Scholar] [CrossRef]
- Kwak, W.J.; Lau, K.C.; Shin, C.D.; Amine, K.; Curtiss, L.A.; Sun, Y.K. A Mo2C/carbon nanotube composite cathode for lithium–oxygen batteries with high energy efficiency and long cycle life. ACS Nano 2015, 9, 4129–4137. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.; Chen, H.; Han, X.; Wu, Y.; He, J.; Han, C.; Yang, G.; Shan, Y. Strongly coupled Mo2C and Ni nanoparticles with in-situ formed interfaces encapsulated by porous carbon nanofibers for efficient hydrogen evolution reaction under alkaline conditions. J. Colloid Interface Sci. 2019, 558, 100–105. [Google Scholar] [CrossRef]
- Kong, M.; Wang, Z.; Wang, W.; Ma, M.; Liu, D.; Hao, S.; Kong, R.; Du, G.; Asiri, A.M.; Yao, Y.; et al. NiCoP nanoarray: A superior pseudocapacitor electrode with high areal capacitance. Chem. A Eur. J. 2017, 23, 4435–4441. [Google Scholar] [CrossRef]
- Wang, C.; Chen, W.; Yuan, D.; Qian, S.; Cai, D.; Jiang, J.; Zhang, S. Tailoring the nanostructure and electronic configuration of metal phosphides for efficient electrocatalytic oxygen evolution reactions. Nano Energy 2020, 7, 104453. [Google Scholar] [CrossRef]
- Surendran, S.; Shanmugapriya, S.; Sivanantham, A.; Shanmugam, S.; Kalai Selvan, R. Electrospun carbon nanofibers encapsulated with NiCoP: A multifunctional electrode for supercapattery and oxygen reduction, oxygen evolution, and hydrogen evolution reactions. Adv. Energy Mater. 2018, 8, 1800555. [Google Scholar] [CrossRef]
- Surendran, S.; Shanmugapriya, S.; Zhu, P.; Yan, C.; Vignesh, R.H.; Lee, Y.S.; Zhang, X.; Selvan, R.K. Hydrothermally synthesised NiCoP nanostructures and electrospun N-doped carbon nanofiber as multifunctional potential electrode for hybrid water electrolyser and supercapatteries. Electrochim. Acta 2019, 296, 1083–1094. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Meng, T.; Pu, Z.; Jin, H.; He, D.; Zhang, J.; Mu, S. Iron oxide and phosphide encapsulated within N, P-doped microporous carbon nanofibers as advanced tri-functional electrocatalyst toward oxygen reduction/evolution and hydrogen evolution reactions and zinc-air batteries. J. Power Sources 2019, 413, 367–375. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakanishi, S.; Hashimoto, K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 2390. [Google Scholar] [CrossRef]
- Chen, S.; Duan, J.; Jaroniec, M.; Qiao, S.Z. Nitrogen and oxygen dual-doped carbon hydrogel film as a substrate-free electrode for highly efficient oxygen evolution reaction. Adv. Mater. 2014, 26, 2925–2930. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, T.R.; Zhou, B.; Cui, Y.T.; Yan, H.; Liu, Z.; Schmitt, F.; Lee, J.; Moore, R.; Chen, Y.; et al. Direct observation of the transition from indirect to direct bandgap in atomically thin epitaxial MoSe 2. Nat. Nanotechnol. 2014, 9, 111. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, G.; Liu, Y.; Xia, J.; Ji, Z.; Shen, X.; Wu, S. Fe3O4-decorated Co9S8 nanoparticles in situ grown on reduced graphene oxide: A new and efficient electrocatalyst for oxygen evolution reaction. Adv. Funct. Mater. 2016, 26, 4712–4721. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Zhou, L.; Cai, X.; Zhan, D.; Hou, M.; Lai, L. Co2P@N,P-codoped carbon nanofiber as a free-standing air electrode for zn–air batteries: Synergy effects of CoNx satellite shells. ACS Appl. Mater. Interfaces 2019, 11, 10364–10372. [Google Scholar] [CrossRef]
- Li, W.; Xiong, D.; Gao, X.; Liu, L. The oxygen evolution reaction enabled by transition metal phosphide and chalcogenide pre-catalysts with dynamic changes. Chem. Commun. 2019, 55, 8744–8763. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, J.; Yan, R.; Du, M.; Wang, Q.; Gao, G.; Wu, J.; Wu, G.; Zhang, M.; Liu, B.; et al. When cubic cobalt sulfide meets layered molybdenum disulfide: A core–shell system toward synergetic electrocatalytic water splitting. Adv. Mater. 2015, 27, 4752–4759. [Google Scholar] [CrossRef]
- Ji, D.; Peng, S.; Fan, L.; Li, L.; Qin, X.; Ramakrishna, S. Thin MoS2 nanosheets grafted MOFs-derived porous Co–N–C flakes grown on electrospun carbon nanofibers as self-supported bifunctional catalysts for overall water splitting. J. Mater. Chem. A 2017, 5, 23898–23908. [Google Scholar] [CrossRef]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, S.; Sinha-Ray, S.; Sinha-Ray, S. Electrospun CNF Supported Ceramics as Electrochemical Catalysts for Water Splitting and Fuel Cell: A Review. Polymers 2020, 12, 238. https://doi.org/10.3390/polym12010238
Verma S, Sinha-Ray S, Sinha-Ray S. Electrospun CNF Supported Ceramics as Electrochemical Catalysts for Water Splitting and Fuel Cell: A Review. Polymers. 2020; 12(1):238. https://doi.org/10.3390/polym12010238
Chicago/Turabian StyleVerma, Sahil, Sumit Sinha-Ray, and Suman Sinha-Ray. 2020. "Electrospun CNF Supported Ceramics as Electrochemical Catalysts for Water Splitting and Fuel Cell: A Review" Polymers 12, no. 1: 238. https://doi.org/10.3390/polym12010238
APA StyleVerma, S., Sinha-Ray, S., & Sinha-Ray, S. (2020). Electrospun CNF Supported Ceramics as Electrochemical Catalysts for Water Splitting and Fuel Cell: A Review. Polymers, 12(1), 238. https://doi.org/10.3390/polym12010238