On the Use of Gallic Acid as a Potential Natural Antioxidant and Ultraviolet Light Stabilizer in Cast-Extruded Bio-Based High-Density Polyethylene Films
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Manufacturing of Films
2.3. Color Measurements
2.4. Mechanical Tests
2.5. Thermal Characterization
2.6. Aging Treatment
2.7. Infrared Spectroscopy
2.8. Microscopy
3. Results and Discussion
3.1. Optical Properties of the GA-Containing Bio-HDPE Films
3.2. Mechanical Properties of the GA-Containing Bio-HDPE Films
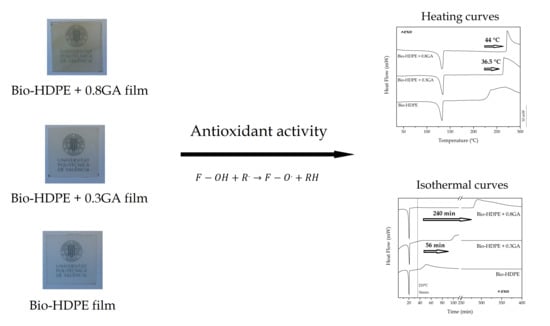
3.3. Thermal Properties of the GA-Containing Bio-HDPE Films
3.4. Chemical Characterization of the GA-Containing Bio-HDPE Films
3.5. UV Light Stability of the GA-Containing Bio-HDPE Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torres-Giner, S.; Gil, L.; Pascual-Ramírez, L.; Garde-Belza, J. Packaging: Food waste reduction. In Encyclopedia of Polymer Applications; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Babu, R.P.; O’connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Li, S.; Jiao, F.; Yuan, Q. Catalytic dehydration of bioethanol to ethylene over tio2/γ-al2o3 catalysts in microchannel reactors. Catal. Today 2007, 125, 111–119. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Jorda-Vilaplana, A.; Balart, R.; Torres-Giner, S. A comparative study on the effect of different reactive compatibilizers on injection-molded pieces of bio-based high-density polyethylene/polylactide blends. J. Appl. Polym. Sci. 2019, 136, 47396. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Torres, A.; Ferrándiz, M.; Fombuena, V.; Balart, R. Antimicrobial activity of metal cation-exchanged zeolites and their evaluation on injection-molded pieces of bio-based high-density polyethylene. J. Food Saf. 2017, 37. [Google Scholar] [CrossRef] [Green Version]
- Vasile, C.; Râpă, M.; Ştefan, M.; Stan, M.; Macavei, S.; Darie-Niţă, R.; Barbu-Tudoran, L.; Vodnar, D.; Popa, E.; Ştefan, R. New pla/zno: Cu/Ag bionanocomposites for food packaging. Express Polym. Lett. 2017, 11, 531–544. [Google Scholar] [CrossRef]
- Carbonell-Verdú, A.; García-García, D.; Jordá, A.; Samper, M.; Balart, R. Development of slate fiber reinforced high density polyethylene composites for injection molding. Compos. Part B Eng. 2015, 69, 460–466. [Google Scholar] [CrossRef]
- Araújo, J.; Waldman, W.; De Paoli, M. Thermal properties of high density polyethylene composites with natural fibres: Coupling agent effect. Polym. Degrad. Stab. 2008, 93, 1770–1775. [Google Scholar] [CrossRef]
- Wang, J.; Du, Z.; Lian, T. Extrusion–calendering process of single-polymer composites based on polyethylene. Polym. Eng. Sci. 2018, 58, 2156–2165. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Fombuena, V.; Balart, R.; Torres-Giner, S. Enhancement of the processing window and performance of polyamide 1010/bio-based high-density polyethylene blends by melt mixing with natural additives. Polym. Int. 2019. [Google Scholar] [CrossRef]
- Gao, X.; Hu, G.; Qian, Z.; Ding, Y.; Zhang, S.; Wang, D.; Yang, M. Immobilization of antioxidant on nanosilica and the antioxidative behavior in low density polyethylene. Polymer 2007, 48, 7309–7315. [Google Scholar] [CrossRef]
- Yu, W.; Reitberger, T.; Hjertberg, T.; Oderkerk, J.; Costa, F.; Englund, V.; Gedde, U.W. Chlorine dioxide resistance of different phenolic antioxidants in polyethylene. Polym. Degrad. Stab. 2015, 111, 1–6. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Haqlwara, A.; Shibata, M.; Ogiso, T. Carcinogenicity of butylated hydroxyanisole in F344 rats. J. Natl. Cancer Inst. 1983, 70, 343–352. [Google Scholar]
- Reddy, V.; Urooj, A.; Kumar, A. Evaluation of antioxidant activity of some plant extracts and their application in biscuits. Food Chem. 2005, 90, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Peltzer, M.; Wagner, J.; Jiménez, A. Thermal characterization of uhmwpe stabilized with natural antioxidants. J. Therm. Anal. Calorim. 2007, 87, 493–497. [Google Scholar] [CrossRef]
- Samper, M.; Fages, E.; Fenollar, O.; Boronat, T.; Balart, R. The potential of flavonoids as natural antioxidants and UV light stabilizers for polypropylene. J. Appl. Polym. Sci. 2013, 129, 1707–1716. [Google Scholar] [CrossRef]
- Tovar, L.; Salafranca, J.; Sánchez, C.; Nerín, C. Migration studies to assess the safety in use of a new antioxidant active packaging. J. Agric. Food Chem. 2005, 53, 5270–5275. [Google Scholar] [CrossRef]
- Kirschweng, B.; Bencze, K.; Sárközi, M.; Hégely, B.; Samu, G.; Hári, J.; Tátraaljai, D.; Földes, E.; Kállay, M.; Pukánszky, B. Melt stabilization of polyethylene with dihydromyricetin, a natural antioxidant. Polym. Degrad. Stab. 2016, 133, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Doudin, K.; Al-Malaika, S.; Sheena, H.; Tverezovskiy, V.; Fowler, P. New genre of antioxidants from renewable natural resources: Synthesis and characterisation of rosemary plant-derived antioxidants and their performance in polyolefins. Polym. Degrad. Stab. 2016, 130, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Martins, S.; Aguilar, C.N.; de la Garza-Rodriguez, I.; Mussatto, S.I.; Teixeira, J.A. Kinetic study of nordihydroguaiaretic acid recovery from larrea tridentata by microwave-assisted extraction. J. Chem. Technol. Biotechnol. 2010, 85, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Vidal, L.; Beltran, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (prunus amygdalus): A multivariate analysis approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kang, N.J.; Lee, B.K.; Lee, K.W.; Lee, H.J. Gallic acid, a metabolite of the antioxidant propyl gallate, inhibits gap junctional intercellular communication via phosphorylation of connexin 43 and extracellular-signal-regulated kinase1/2 in rat liver epithelial cells. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2008, 638, 175–183. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. Bioactive multilayer polylactide films with controlled release capacity of gallic acid accomplished by incorporating electrospun nanostructured coatings and interlayers. Appl. Sci. 2019, 9, 533. [Google Scholar] [CrossRef] [Green Version]
- Agüero, A.; Morcillo, M.d.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the influence of the reprocessing cycles on the final properties of polylactide pieces obtained by injection molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef] [Green Version]
- Castro, D.; Ruvolo-Filho, A.; Frollini, E. Materials prepared from biopolyethylene and curaua fibers: Composites from biomass. Polym. Test. 2012, 31, 880–888. [Google Scholar] [CrossRef]
- Paradkar, R.; Sakhalkar, S.; He, X.; Ellison, M. Estimating crystallinity in high density polyethylene fibers using online raman spectroscopy. J. Appl. Polym. Sci. 2003, 88, 545–549. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Goodwin, C.; Issenhuth, S.; Burdick, D. The antioxidant role of α-tocopherol in polymers II. Melt stabilising effect in polypropylene. Polym. Degrad. Stab. 1999, 64, 145–156. [Google Scholar] [CrossRef]
- Fenollar, O.; García, D.; Sánchez, L.; López, J.; Balart, R. Optimization of the curing conditions of pvc plastisols based on the use of an epoxidized fatty acid ester plasticizer. Eur. Polym. J. 2009, 45, 2674–2684. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and antioxidant performance of various essential oils and natural extracts and their incorporation into biowaste derived poly(3-hydroxybutyrate-co-3-hydroxyvalerate) layers made from electrospun ultrathin fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef] [Green Version]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun antimicrobial films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) containing eugenol essential oil encapsulated in mesoporous silica nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Møller, J.K.S.; Bertelsen, G.; Skibsted, L.H. Photooxidation of nitrosylmyoglobin at low oxygen pressure. Quantum yields and reaction stoechiometries. Meat Sci. 2002, 60, 421–425. [Google Scholar] [CrossRef]
- Zhong, Y.; Janes, D.; Zheng, Y.; Hetzer, M.; De Kee, D. Mechanical and oxygen barrier properties of organoclay-polyethylene nanocomposite films. Polym. Eng. Sci. 2007, 47, 1101–1107. [Google Scholar] [CrossRef]
- Ferrero, B.; Fombuena, V.; Fenollar, O.; Boronat, T.; Balart, R. Development of natural fiber-reinforced plastics (NFRP) based on biobased polyethylene and waste fibers from posidonia oceanica seaweed. Polym. Compos. 2015, 36, 1378–1385. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded pla by controlling crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Cleymand, F.; Leconte, S.; Falher, T.; Desobry, S. Effects of synthetic phenolic antioxidants on physical, structural, mechanical and barrier properties of poly lactic acid film. Carbohydr. Polym. 2012, 87, 1763–1773. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Akhtar, M.J.; Cleymand, F.; Desobry, S. Structural, mechanical and barrier properties of active pla–antioxidant films. J. Food Eng. 2012, 110, 380–389. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and α-tocopherol. Food Hydrocoll. 2008, 22, 449–458. [Google Scholar] [CrossRef]
- Chirinos Padrón, A.J.; Colmenares, M.A.; Rubinztain, Z.; Albornoz, L.A. Influence of additives on some physical properties of high density polyethylene-i. Commercial antioxidants. Eur. Polym. J. 1987, 23, 723–727. [Google Scholar] [CrossRef]
- Chirinos Padrón, A.J.; Rubinztain, Z.; Colmenares, M.A. Influence of additives on some physical properties of high density polyethylene-II. Commercial u.V. Stabilizers. Eur. Polym. J. 1987, 23, 729–732. [Google Scholar] [CrossRef]
- López-Naranjo, E.J.; Alzate-Gaviria, L.M.; Hernández-Zárate, G.; Reyes-Trujeque, J.; Cupul-Manzano, C.V.; Cruz-Estrada, R.H. Effect of biological degradation by termites on the flexural properties of pinewood residue/recycled high-density polyethylene composites. J. Appl. Polym. Sci. 2013, 128, 2595–2603. [Google Scholar] [CrossRef]
- Xu, T.; Lei, H.; Xie, C. The effect of nucleating agent on the crystalline morphology of polypropylene (PP). Mater. Des. 2003, 24, 227–230. [Google Scholar] [CrossRef]
- López-de-Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem. 2012, 131, 1376–1384. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Carbonell-Verdú, A.; Samper, M.-D.; Pop, A.; Garcia-Sanoguera, D. Development and characterization of a new natural fiber reinforced thermoplastic (NFRP) with cortaderia selloana (pampa grass) short fibers. Compos. Sci. Technol. 2017, 145, 1–9. [Google Scholar] [CrossRef]
- Dopico-García, M.; Castro-López, M.; López-Vilariño, J.; González-Rodríguez, M.; Valentao, P.; Andrade, P.; García-Garabal, S.; Abad, M. Natural extracts as potential source of antioxidants to stabilize polyolefins. J. Appl. Polym. Sci. 2011, 119, 3553–3559. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Ning, M.; Zhang, H. Synthesis and antioxidant activities in polyolefin of dendritic antioxidants with hindered phenolic groups and tertiary amine. J. Appl. Polym. Sci. 2012, 124, 4127–4135. [Google Scholar] [CrossRef]
- Koontz, J.L.; Marcy, J.E.; O’Keefe, S.F.; Duncan, S.E.; Long, T.E.; Moffitt, R.D. Polymer processing and characterization of LLDPE films loaded with α-tocopherol, quercetin, and their cyclodextrin inclusion complexes. J. Appl. Polym. Sci. 2010, 117, 2299–2309. [Google Scholar] [CrossRef]
- Brewer, M. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef]
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Medicinal importance of gallic acid and its ester derivatives: A patent review. Pharm. Pat. Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid b-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Jana, R.N.; Mukunda, P.G.; Nando, G.B. Thermogravimetric analysis of compatibilized blends of low density polyethylene and poly(dimethyl siloxane) rubber. Polym. Degrad. Stab. 2003, 80, 75–82. [Google Scholar] [CrossRef]
- Montanes, N.; Garcia-Sanoguera, D.; Segui, V.; Fenollar, O.; Boronat, T. Processing and characterization of environmentally friendly composites from biobased polyethylene and natural fillers from thyme herbs. J. Polym. Environ. 2018, 26, 1218–1230. [Google Scholar] [CrossRef]
- Zeinalov, E.B.; Koßmehl, G. Fullerene C60 as an antioxidant for polymers. Polym. Degrad. Stab. 2001, 71, 197–202. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Dominici, F.; Fortunati, E.; Giovanale, G.; Balestra, G.; Torre, L. Effect of gallic acid and umbelliferone on thermal, mechanical, antioxidant and antimicrobial properties of poly(vinyl alcohol-co-ethylene) films. Polym. Degrad. Stab. 2018, 152, 162–176. [Google Scholar] [CrossRef]
- Cerruti, P.; Malinconico, M.; Rychly, J.; Matisova-Rychla, L.; Carfagna, C. Effect of natural antioxidants on the stability of polypropylene films. Polym. Degrad. Stab. 2009, 94, 2095–2100. [Google Scholar] [CrossRef]
- Ambrogi, V.; Cerruti, P.; Carfagna, C.; Malinconico, M.; Marturano, V.; Perrotti, M.; Persico, P. Natural antioxidants for polypropylene stabilization. Polym. Degrad. Stab. 2011, 96, 2152–2158. [Google Scholar] [CrossRef]
- España, J.; Fages, E.; Moriana, R.; Boronat, T.; Balart, R. Antioxidant and antibacterial effects of natural phenolic compounds on green composite materials. Polym. Compos. 2012, 33, 1288–1294. [Google Scholar] [CrossRef] [Green Version]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, F.; Luo, Y.; Ma, L.; Kou, X.; Huang, K. Antioxidant activity of a water-soluble polysaccharide purified from pteridium aquilinum. Carbohydr. Res. 2009, 344, 217–222. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Sugimoto, M.; Shimada, A.; Kudoh, H.; Tamura, K.; Seguchi, T. Product analysis for polyethylene degradation by radiation and thermal ageing. Radiat. Phys. Chem. 2013, 82, 69–73. [Google Scholar] [CrossRef]
- Markarian, S.A.; Zatikyan, A.L.; Bonora, S.; Fagnano, C. Raman and ft ir atr study of diethylsulfoxide/water mixtures. J. Mol. Struct. 2003, 655, 285–292. [Google Scholar] [CrossRef]
- Gil-Longo, J.; González-Vázquez, C. Vascular pro-oxidant effects secondary to the autoxidation of gallic acid in rat aorta. J. Nutr. Biochem. 2010, 21, 304–309. [Google Scholar] [CrossRef]
- Qin, H.; Zhao, C.; Zhang, S.; Chen, G.; Yang, M. Photo-oxidative degradation of polyethylene/montmorillonite nanocomposite. Polym. Degrad. Stab. 2003, 81, 497–500. [Google Scholar] [CrossRef]
- Grigoriadou, I.; Paraskevopoulos, K.; Chrissafis, K.; Pavlidou, E.; Stamkopoulos, T.-G.; Bikiaris, D. Effect of different nanoparticles on HDPE UV stability. Polym. Degrad. Stab. 2011, 96, 151–163. [Google Scholar] [CrossRef]
- Scott, G. The antioxidant role of uv stabilisers. Pure Appl. Chem. 1980, 52, 365–387. [Google Scholar] [CrossRef]
- Du, H.; Wang, W.; Wang, Q.; Zhang, Z.; Sui, S.; Zhang, Y. Effects of pigments on the UV degradation of wood-flour/HDPE composites. J. Appl. Polym. Sci. 2010, 118, 1068–1076. [Google Scholar] [CrossRef]







| Film | L* | a* | b* | ΔEab* |
|---|---|---|---|---|
| Bio-HDPE | 82.9 ± 1.0 | −1.9 ± 0.1 | −2.8 ± 0.3 | - |
| Bio-HDPE + 0.3GA | 75.3 ± 0.9 | −0.7 ± 0.3 | 8.8 ± 0.4 | 13.9 ± 0.9 |
| Bio-HDPE + 0.8GA | 70.6 ± 0.5 | −0.1 ± 0.1 | 10.9 ± 0.2 | 18.5 ± 0.4 |
| Film | Etensile (MPa) | σmax (MPa) | εb (%) |
|---|---|---|---|
| Bio-HDPE | 292.5 ± 22.1 | 21.3 ± 1.2 | 45.2 ± 3.5 |
| Bio-HDPE + 0.3GA | 222.1 ± 24.2 | 20.1 ± 0.6 | 18.6 ± 2.1 |
| Bio-HDPE + 0.8GA | 243.6 ± 31.5 | 20.8 ± 0.9 | 20.2 ± 2.3 |
| Film | Tm (°C) | ΔHm (J·g−1) | XC (%) | OTT (°C) | OIT (min) |
|---|---|---|---|---|---|
| Bio-HDPE | 132.1 ± 0.3 | 160.6 ± 1.5 | 54.8± 0.8 | 226.3 ± 1.5 | 4.9 ± 0.3 |
| Bio-HDPE + 0.3GA | 132.4 ± 0.5 | 156.5 ± 1.4 | 53.4± 0.7 | 262.8 ± 2.1 | 60.8 ± 0.5 |
| Bio-HDPE + 0.8GA | 132.2 ± 0.7 | 152.7 ± 2.0 | 52.0 ± 0.9 | 270.2 ± 1.9 | 244.7 ± 1.0 |
| Film | Tonset (°C) | Tdeg (°C) | Residual mass (%) |
|---|---|---|---|
| Bio-HDPE | 256.9 ± 1.8 | 427.8 ± 1.3 | 0.22 ± 0.05 |
| Bio-HDPE + 0.3GA | 283.9 ± 2.0 | 442.9 ± 1.1 | 0.20 ± 0.04 |
| Bio-HDPE + 0.8GA | 291.6 ± 2.1 | 444.6 ± 1.2 | 0.17± 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiles-Carrillo, L.; Montava-Jordà, S.; Boronat, T.; Sammon, C.; Balart, R.; Torres-Giner, S. On the Use of Gallic Acid as a Potential Natural Antioxidant and Ultraviolet Light Stabilizer in Cast-Extruded Bio-Based High-Density Polyethylene Films. Polymers 2020, 12, 31. https://doi.org/10.3390/polym12010031
Quiles-Carrillo L, Montava-Jordà S, Boronat T, Sammon C, Balart R, Torres-Giner S. On the Use of Gallic Acid as a Potential Natural Antioxidant and Ultraviolet Light Stabilizer in Cast-Extruded Bio-Based High-Density Polyethylene Films. Polymers. 2020; 12(1):31. https://doi.org/10.3390/polym12010031
Chicago/Turabian StyleQuiles-Carrillo, Luis, Sergi Montava-Jordà, Teodomiro Boronat, Chris Sammon, Rafael Balart, and Sergio Torres-Giner. 2020. "On the Use of Gallic Acid as a Potential Natural Antioxidant and Ultraviolet Light Stabilizer in Cast-Extruded Bio-Based High-Density Polyethylene Films" Polymers 12, no. 1: 31. https://doi.org/10.3390/polym12010031
APA StyleQuiles-Carrillo, L., Montava-Jordà, S., Boronat, T., Sammon, C., Balart, R., & Torres-Giner, S. (2020). On the Use of Gallic Acid as a Potential Natural Antioxidant and Ultraviolet Light Stabilizer in Cast-Extruded Bio-Based High-Density Polyethylene Films. Polymers, 12(1), 31. https://doi.org/10.3390/polym12010031