Variations in Wettability and Interfacial Tension during Alkali–Polymer Application for High and Low TAN Oils
Abstract
:1. Introduction
2. Materials and Methods
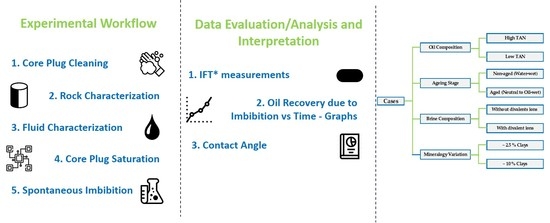
2.1. Methodology and Approach
- For a potential field pilot, we chose to use oil from a defined reservoir; hence, oils with high and low TAN values were selected and further studied.
- Interfacial tension was measured as a function of time over 300 min at a temperature of 60 °C for the defined chemicals and oils. The evaluation was based on the assessment proposed by Sharma et al. [18].
- Core plugs were saturated to determine irreducible water (Swirr) and initial oil saturation (Soi). Permeability to water and oil were defined during the saturation process and cross-analyzed.
- Two groups of cores were considered during the process, aged and non-aged, to assess any wettability shift at reservoir temperature. Core aging at reservoir temperature for around 800 h was reported to lead to a wettability shift from a purely water-wet state of the cleaned core samples to a neutral or oil wet state [3,34,35].
- Amott spontaneous imbibition experiments were performed for different defined cases considering the influence of TAN, core plug aging, brine composition, and clay rock content. The results were assessed by a detailed analysis of oil production with time dependency and final oil recovery.
2.2. Fluids and Core Sample Data
2.2.1. Brines
2.2.2. Oil Samples
2.2.3. Chemical Solutions
2.2.4. Core Samples
- The permeability of the core plugs resemble the permeability ranges of the 16 TH formation where alkali–polymer flooding is under investigation for possible implementation [16].
- The core plugs are highly similar in their properties (their observed ranges and differences in porosity and permeability values are narrow).
- The rock mineralogy is simple, thereby simplifying any additional uncertainties during the experimental analysis and facilitating data acquisition.
2.2.5. Keuper Core
2.3. Interfacial Tension (IFT) Evaluations
- Different algorithms considered for the measurement based on drop shape and fluid characteristics.Two different algorithms were available for the measurements (Vonnegut and Young–Laplace). For systems with an interfacial tension greater than 1 mN/m (test water and polymer solutions), the Vonnegut algorithm could not be applied because of the oil droplet shape. Therefore, the Young–Laplace algorithm was used. At the same time, for systems with an IFT lower than 1 mN/m (alkali and alkali–polymer solutions), the Young–Laplace method could not be used because of the highly elongated shape of the oil droplet. Hence, the Vonnegut method was implemented in these cases.
- Lack of proper temperature stabilization leading to a malfunction of the device.From time to time, the recording camera stopped responding, which led to missing data for this period until the camera was set up again.
- The collapse and/or conjunction of multiple oil droplets.Some errors due to collision and separation of multiple oil droplets were observed. In the case of low IFT (alkali and alkali–polymer solutions), the droplets were especially unstable over time. Due to separation/collision of the droplets, the volume changed and, consequently, the IFT measurement was affected.
2.4. Contact Angle Measurements
2.5. Spontaneous Imbibition Evaluations
- Oil production with time dependency (revealing how capillary forces will perform over time);
- Final oil recovery, which represents the total amount of oil trapped in the system.
3. Results and Discussion
3.1. Interfacial Tension Observations
3.2. Contact Angle Results
3.3. Spontaneous Imbibition Observations
3.3.1. Non-Aged Nordhorn Core Plugs—Oil Type Comparison
3.3.2. Nordhorn Core Plugs—Influence of Aging
3.3.3. Nordhorn Core Plugs—Brine Composition
3.3.4. Nordhorn and Keuper Core Plugs—Influence of Mineralogy
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheng, J.J. (Ed.) “Copyright”. In Modern Chemical Enhanced Oil Recovery; Gulf Professional Publishing: Boston, MA, USA, 2011; p. iv. [Google Scholar]
- Arekhov, V.; Hincapie, R.E.; Clemens, T. Wettability Changes and Interfacial Tension Reduction in Alkali Polymer Flooding of High and Low Tan Number Oils. In Proceedings of the SPE Europec featured at 82nd EAGE Conference and Exhibition, Amsterdam, The Netherlands, 8–11 December 2020. [Google Scholar] [CrossRef]
- McPhee, C.; Reed, J.; Zubizarreta, I. Chapter 1—Best Practice in Coring and Core Analysis. In Developments in Petroleum Science 64; McPhee, C., Reed, J., Zubizarreta, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–15. [Google Scholar]
- Khaleel, O.; Teklu, T.W.; Alameri, W.; Abass, H.; Kazemi, H. Wettability Alteration of Carbonate Reservoir Cores—Laboratory Evaluation Using Complementary Techniques. SPE Reserv. Eval. Eng. 2019, 22, 911–922. [Google Scholar] [CrossRef]
- Tagavifar, M.; Balhoff, M.; Mohanty, K.; Pope, G.A. Dynamics of Low-Interfacial-Tension Imbibition in Oil-Wet Carbonates. SPE J. 2019, 24, 1.092–1.107. [Google Scholar] [CrossRef]
- Juarez-Morejon, J.L.; Bertin, H.; Omari, A.; Hamon, G.; Cottin, C.; Bourdarot, G.; Morel, D. Spontaneous Imbibition as Indicator of Wettability Change During Polymer Flooding. In Proceedings of the IOR 2017—19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017; pp. 1–10. [Google Scholar] [CrossRef]
- Al-Menhali, A.; Niu, B.; Krevor, S. Capillarity and wetting of carbon dioxide and brine during drainage in Berea sandstone at reservoir conditions. Water Resour. Res. 2015, 51, 7895–7914. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Dong, M.; Shirif, E. Study of Alkaline/Polymer Flooding for Heavy-Oil Recovery Using Channeled Sandpacks. SPE Reserv. Eval. Eng. 2011, 14, 310–319. [Google Scholar] [CrossRef]
- Alzahid, Y.; Mostaghimi, P.; Warkiani, M.E.; Armstrong, R.T.; Joekar-Niasar, V.; Karadimitriou, N. Alkaline Surfactant Polymer Flooding: What Happens at the Pore Scale? In Proceedings of the SPE Europec featured at 79th EAGE Conference and Exhibition, Paris, France, 12–15 June 2017. [Google Scholar] [CrossRef]
- Sun, C.; Guo, H.; Li, Y.; Jiang, G.; Ma, R. Alkali Effect on Alkali-Surfactant-Polymer (ASP) Flooding Enhanced Oil Recovery Performance: Two Large-Scale Field Tests’ Evidence. J. Chem. 2020, 2020, 2829565. Available online: https://www.hindawi.com/journals/jchem/2020/2829565/ (accessed on 20 August 2020). [CrossRef]
- Sheng, J.J. Chapter 7—Alkaline-Polymer Flooding. In Enhanced Oil Recovery Field Case Studies; Sheng, J.J., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2013; pp. 169–178. [Google Scholar]
- Sheng, J.J. Critical review of alkaline-polymer flooding. J Pet. Explor. Prod. Technol. 2017, 7, 147–153. [Google Scholar] [CrossRef]
- Maneeintr, K.; Meekoch, T.; Jongkittinarukorn, K.; Boonpramote, T. Interfacial Tension Measurement for Alkaline-Polymer Flooding Application for Oil from Fang Oilfield, Thailand. Chem. Eng. Trans. 2020, 78, 487–492. [Google Scholar] [CrossRef]
- Aitkulov, A.; Luo, H.; Lu, J.; Mohanty, K.K. Alkali–Cosolvent–Polymer Flooding for Viscous Oil Recovery: 2D Evaluation. 21 June 2017. Available online: https://pubs.acs.org/doi/abs/10.1021/acs.energyfuels.7b00790 (accessed on 20 August 2020).
- Schumi, B.; Clemens, T.; Wegner, J.; Ganzer, L.; Kaiser, A.; Hincapie, R.E.; Leitenmüller, V. Alkali/Cosolvent/Polymer Flooding of High-TAN Oil: Using Phase Experiments, Micromodels, and Corefloods for Injection-Agent Selection. SPE Reserv. Eval. Eng. 2019. [Google Scholar] [CrossRef]
- Mortazavi, E.; Masihi, M.; Ghazanfari, M.H. An Influence of Polymer-Alkaline and Nanoparticles as Chemical Additives on the Immiscible Displacement and Phase Relative Permeability. Iran. J. Oil Gas Sci. Technol. 2016, 5, 14–31. [Google Scholar] [CrossRef]
- Schechter, D.S.; Zhou, D.; Orr, F.M. Low IFT drainage and imbibition. J. Pet. Sci. Eng. 1994, 11, 283–300. [Google Scholar] [CrossRef]
- Sharma, M.M.; Jang, L.K.; Yen, T.F. Transient Interfacial Tension Behavior of Crude-Oil/Caustic Interfaces. SPE Reserv. Eng. 1989, 4, 228–236. [Google Scholar] [CrossRef]
- Skripkin, A.G.; Kuznetsov, I.A.; Volokitin, Y.E.; Chmuzh, I. Experimental studies of oil recovery after Alkali-Surfactant-Polymer (ASP) flooding with West Salym cores. In Proceedings of the SPE Russian Oil and Gas Exploration and Production Technical Conference and Exhibition, Moscow, Russia, 16–18 October 2012. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, C.; Wu, G.; Yuan, H.; Yu, L.; Tian, G. A Study of Microscopic Flooding Mechanism of Surfactant/Alkali/Polymer. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 19–22 April 1998. [Google Scholar] [CrossRef]
- Arihara, N.; Yoneyama, T.; Akita, Y.; XiangGuo, L. Oil Recovery Mechanisms of Alkali-Surfactant-Polymer Flooding. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 20–22 April 1999. [Google Scholar] [CrossRef]
- Ehrlich, R.; Hasiba, H.H.; Raimondi, P. Alkaline Waterflooding for Wettability Alteration-Evaluating a Potential Field Application. J. Pet. Technol. 1974, 26, 1335–1343. [Google Scholar] [CrossRef]
- Jennings, H.Y.J.; Johnson, C.E.J.; McAuliffe, C.D. A Caustic Waterflooding Process for Heavy Oils. J. Pet. Technol. 1974, 26, 1344–1352. [Google Scholar] [CrossRef]
- Emery, L.W.; Mungan, N.; Nicholson, R.W. Caustic Slug Injection in the Singleton Field. J. Pet. Technol. 1970, 22, 1569–1576. [Google Scholar] [CrossRef]
- Yuan, S.; Yang, H.; Shen, K.; Yang, P. Effects of Important Factors on Alkali/Surfactant/Polymer Flooding. In Proceedings of the SPE International Oil and Gas Conference and Exhibition in China, Beijing, China, 2–6 November 1998. [Google Scholar] [CrossRef]
- De Zabala, E.F.; Radke, C.J. A Nonequilibrium Description of Alkaline Waterflooding. SPE Reserv. Eng. 1986, 1, 29–43. [Google Scholar] [CrossRef]
- Wei, J.; Tao, J.; Xin, S.; Zhang, Q.; Zhang, X.; Wang, X. A Study on Alkali Consumption Regularity in Minerals of Reservoirs During Alkali(NaoH)/Surfactant/Polymer Flooding in Daqing Oilfield. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 19–21 July 2011. [Google Scholar] [CrossRef]
- Sheng, J.J.; Leonhardt, B.; Azri, N. Status of Polymer-Flooding Technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- Abe, A. Relative Permeability and Wettability Implications of Dilute Surfactants at Reservoir Conditions. Master’s Thesis. 2005. Available online: https://digitalcommons.lsu.edu/gradschool_theses/2660 (accessed on 20 August 2020).
- Awolayo, A.N.; Sarma, H.K.; Nghiem, L.X. Brine-Dependent Recovery Processes in Carbonate and Sandstone Petroleum Reservoirs: Review of Laboratory-Field Studies, Interfacial Mechanisms and Modeling Attempts. Energies 2018, 11, 3020. [Google Scholar] [CrossRef] [Green Version]
- Mohnot, S.M.; Bae, J.H.; Foley, W.L. A Study of Mineral/Alkali Reactions. SPE Reserv. Eng. 1987, 2, 653–663. [Google Scholar] [CrossRef]
- Leitenmueller, V.; Toumi, O.; Hofstaetter, H.; Clemens, T. Microemulsion Formation & Its Effect on Rheology Using Carbonate-Based Alkalis for AP or ASP Floods in the Matzen Field. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26–28 March 2018. [Google Scholar] [CrossRef]
- Neubauer, E.; Hincapie, R.E.; Borovina, A.; Biernat, M.; Clemens, T.; Ahmad, Y.K. Influence of Nanofluids on Wettability Changes and Interfacial Tension Reduction. In Proceedings of the SPE Europec featured at 82nd EAGE Conference and Exhibition, Amsterdam, The Netherlands, 8–11 December 2020. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Ganzer, L. Unlocking the Effects of Fluid Optimization on Remaining Oil Saturation for the Combined Sulfate-Modified Water and Polymer Flooding. Energies 2020, 13, 3049. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Langanke, N.; Ganzer, L.; Jaeger, P. Coupling Microfluidics Data with Core Flooding Experiments to Understand Sulfonated/Polymer Water Injection. Polymers 2020, 12, 1227. [Google Scholar] [CrossRef]
- Rupprecht, B.J.; Sachsenhofer, R.F.; Zach, C.; Bechtel, A.; Gratzer, R.; Kucher, F. Oil and gas in the Vienna Basin: Hydrocarbon generation and alteration in a classical hydrocarbon province. Pet. Geosci. 2019, 25, 3–29. [Google Scholar] [CrossRef]
- Chevalier, T.; Labaume, J.; Delbos, A.; Clemens, T.; Waeger, V.M.; Bourbiaux, B.; Fleury, M. A novel experimental approach for studying spontaneous imbibition processes with alkaline solutions. E3s Web Conf. 2019, 89, 04004. [Google Scholar] [CrossRef]
- Arnold, P. Experimental Investigation of interfacial Tension for Alkaline Flooding. Master’s Thesis, University of Leoben, Leoben, Austria, 2018. [Google Scholar]
- Tahir, M.; Hincapie, R.E.; Foedisch, H.; Strobel, G.-J.; Ganzer, L. Potential Benefits of Fluid Optimization for Combined Smart-Water and Polymer Flooding: Impact on Remaining Oil Saturation. In Proceedings of the SPE Russian Petroleum Technology Conference, Moscow, Russia, 22–24 October 2019. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Gaol, C.; Saefken, S.; Ganzer, L. Describing The Flow Behavior Of Smart Water In Micromodels With Wettability Modified Pore Structures. Soc. Pet. Eng. 2020. [Google Scholar] [CrossRef]
- Tahir, M. Experimental Investigation of Sulfate-Modified Water and Polymer Flooding for Enhanced Oil Recovery. Ph.D. Thesis, TU Clausthal, Clausthal-Zellerfeld, Germany, 2020. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Foedisch, H.; Abdullah, H.; Ganzer, L. Impact of Sulphates Presence During Application of Smart Water Flooding Combined with Polymer Flooding. In Proceedings of the SPE Europec Featured at 80th EAGE Conference and Exhibition, Copenhagen, Denmark, 11–14 June 2018. [Google Scholar] [CrossRef]
- Mohamed, M.I.; Alvarado, V. Smart Water Flooding in Berea Sandstone at Low Temperature: Is Wettability Alteration the Sole Mechanism at Play? In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 9–11 October 2017. [Google Scholar] [CrossRef]
- Al-Saedi, H.N.; Flori, R.E.; Al-Jaberi, S.K. Eliminate the role of clay in sandstone: EOR low salinity water flooding. J. Pet. Explor. Prod. Technol. 2019, 9, 1475–1483. [Google Scholar] [CrossRef] [Green Version]
- Al-Saedi, H.; Flori, R.E.; Mortadha, A. Investigation of Smart Water Flooding in Sandstone Reservoirs: Experimental and Simulation Study Part 2. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 12–15 November 2018. [Google Scholar] [CrossRef]
- Skauge, T.; Djurhuus, K.; Reichenbach-Klinke, R. Visualization of Heavy Oil Mobilization by Associative Polymer. In Proceedings of the SPE Europec featured at 80th EAGE Conference and Exhibition, Copenhagen, Denmark, 11–14 June 2018. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Ganzer, L. Influence of Sulfate Ions on the Combined Application of Modified Water and Polymer Flooding—Rheology and Oil Recovery. Energies 2020, 13, 2356. [Google Scholar] [CrossRef]
- Haagh, M.E.J.; Siretanu, I.; Duits, M.H.G.; Mugele, F. Salinity-Dependent Contact Angle Alteration in Oil/Brine/Silicate Systems: The Critical Role of Divalent Cations. Langmuir 2017, 33, 3349–3357. [Google Scholar] [CrossRef]
- Vijapurapu, C.S.; Rao, D.N. Effect of Brine Dilution and Surfactant Concentration on Spreading and Wettability. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5–7 February 2003. [Google Scholar] [CrossRef]





























| No. | Chemicals | Experimental Approach | Recovery Mechanisms | Insightful Outcome | Reference |
|---|---|---|---|---|---|
| 1 | A/P/AP | Channeled sandpack flooding | Improved mobility ratio | Plugging and diverted flow | Wu et al. [8] |
| 2 | A/AP/ASP | Microfluidics chip | Viscosity of displacing fluid + pore-scale morphology | At same capillary number + mobility number | Alzahir et al. [9] |
| 3 | ASP | Daqing oilfield tests | Ultra-low interfacial tension (IFT) under oil-wet state | Weak alkali in ASP worked better than strong alkali in ASP | Sun et al. [10] |
| 4 | AP | Book chapter with field applications | Improved sweep efficiency + in situ soap generation | Low adsorption + better injectivity | Sheng.J. [11] |
| 5 | AP | Field projects + laboratory + simulation Summary | Polymer mobility control + soap improved displacement efficiency | Mixed outcomes from field tests | Sheng, J. [12] |
| 6 | AP | IFT study for Fang oilfield test | Lower IFT + improved mobility ratio | Effect of pressure, temperature, and salinity on IFT | Maneeintr et al. [13] |
| 7 | ACP | Sandpack slap flooding + simulation | Slug viscosity + ultra-low IFT | Optimum initiation time in heavy oil | Aitkulov et al. [14] |
| 8 | AP + Nanoparticles | Unsteady state displacement experiments | In situ soap generation, pressure increment | Higher oil relative permeability values | Mortazavi et al. [15] |
| 9 | ACP/AP | Micromodels, core flood | Improved sweep efficiency + in situ soap generation | Low adsorption, better sweep efficiency and cost efficiency | Schumi et al. [16] |
| Parameter | Units | Test Water | Formation Brine | ||
|---|---|---|---|---|---|
| Mean | SD * | Mean | SD * | ||
| Density, 25 °C | Kg/m3 | 1011.700 | 0.000 | 1012.000 | 0.000 |
| Density, 60 °C | 997.100 | 0.100 | 1012.000 | 0.000 | |
| Shear Viscosity, 25 °C | mPa∙s | 0.964 | 0.032 | 0.954 | 0.030 |
| Shear Viscosity, 60 °C | 0.571 | 0.023 | 0.592 | 0.054 | |
| Components | Units | Formation Brine | Test Water | ||
| NaCl | g/L | 19.750 | 18.960 | ||
| NaHCO3 | g/L | - | 1.850 | ||
| CaCl2·2H2O | g/L | 0.400 | - | ||
| MgCl2·6H2O | g/L | 0.660 | - | ||
| NH4Cl | g/L | 0.170 | - | ||
| SrCl2·6H2O | g/L | 0.060 | - | ||
| BaCl2·2H2O | g/L | 0.030 | - | ||
| Parameter | Units | 16 TH | St. Ulrich | ||
|---|---|---|---|---|---|
| Mean | SD * | Mean | SD * | ||
| Density, 25 °C | Kg/m3 | 907.30 | 0.10 | 866.60 | 0.20 |
| Density, 60 °C | 884.30 | 0.20 | 842.60 | 0.60 | |
| Shear Viscosity, 60 °C | mPa∙s | 11.90 | 0.10 | 6.00 | 0.10 |
| TAN (total acid number) | mg KOH/g | 1.61 | 0.18 | 0.17 | 0.08 |
| Parameter | Units | Alkali | Polymer | AP Solution | |||
|---|---|---|---|---|---|---|---|
| Mean | SD * | Mean | SD * | Mean | SD * | ||
| Density, 25 °C | Kg/cm3 | 1020.00 | 0.000 | 1013.00 | 0.00 | 1018.70 | 0.100 |
| Density, 60 °C | 1005.20 | 0.10 | 998.50 | 0.00 | 1004.20 | 0.000 | |
| Viscosity @7,94 s−1, 25 °C | mPa∙s | 0.99 | 0.02 | 27.78 | 0.002 | 25.910 | 0.001 |
| Viscosity @7,94 s−1, 60 °C | 0.55 | 0.02 | 19.54 | 0.002 | 18.050 | 0.002 | |
| deviation | |||||||
| Parameter | Units | Nordhorn | Keuper | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Length | cm | 8.010 | 0.110 | 8.120 | 0.090 |
| Diameter | 2.960 | 0.010 | 2.980 | 0.010 | |
| Bulk volume | cm3 | 54.420 | 0.870 | 55.760 | 0.730 |
| Pore volume | 13.070 | 0.260 | 12.750 | 0.220 | |
| Grain volume | kg/m3 | 41.360 | 0.700 | 42.980 | 0.670 |
| Porosity | % | 23.960 | 0.350 | 22.790 | 0.340 |
| N2 permeability | mD | 2313.000 | 162.000 | 1327.000 | 186.000 |
| Water (test water) permeability | 1500.000 | 190.000 | 902.000 | 198.000 | |
| Irreducible water saturation | % | 25.600 | 4.000 | 32.500 | 4.400 |
| Oil permeability | mD | 314.000 | 79.000 | 178.000 | 51.000 |
| End-point oil relative permeability | - | 0.210 | 0.060 | 0.200 | 0.070 |
| Initial oil saturation | % | 74.400 | 4.000 | 67.500 | 4.400 |
| Core | Time (Min) | Contact Angle—16 TH | Contact Angle—St. Ulrich | ||||
|---|---|---|---|---|---|---|---|
| TW * | Alkali | Alkali–Polymer | TW * | Alkali | Alkali–Polymer | ||
| Nordhorn | 0 | 60.0 | 57.8 | 43.7 | 62.7 | 38.1 | 61.8 |
| 300 | 58.7 | 57.2 | 57.8 | 60.7 | 42.7 | 59.8 | |
| Keuper | 0 | 49.1 | 53.3 | 60.7 | 45.2 | 51.9 | 34.6 |
| 300 | 50.4 | 54.2 | 56.1 | 50.0 | 47.8 | 55.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arekhov, V.; Hincapie, R.E.; Clemens, T.; Tahir, M. Variations in Wettability and Interfacial Tension during Alkali–Polymer Application for High and Low TAN Oils. Polymers 2020, 12, 2241. https://doi.org/10.3390/polym12102241
Arekhov V, Hincapie RE, Clemens T, Tahir M. Variations in Wettability and Interfacial Tension during Alkali–Polymer Application for High and Low TAN Oils. Polymers. 2020; 12(10):2241. https://doi.org/10.3390/polym12102241
Chicago/Turabian StyleArekhov, Vladislav, Rafael E. Hincapie, Torsten Clemens, and Muhammad Tahir. 2020. "Variations in Wettability and Interfacial Tension during Alkali–Polymer Application for High and Low TAN Oils" Polymers 12, no. 10: 2241. https://doi.org/10.3390/polym12102241
APA StyleArekhov, V., Hincapie, R. E., Clemens, T., & Tahir, M. (2020). Variations in Wettability and Interfacial Tension during Alkali–Polymer Application for High and Low TAN Oils. Polymers, 12(10), 2241. https://doi.org/10.3390/polym12102241