Axial Orientation of Co-Crystalline Phases of Poly(2,6-Dimethyl-1,4-Phenylene)Oxide Films
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Film Preparation
2.2. Characterization Techniques
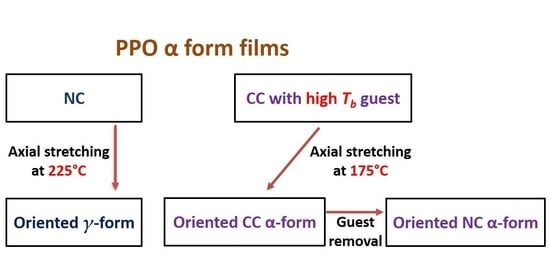
3. Results and Discussion
3.1. Sorption of Toluene in Axially Oriented NC PPO Blend Films with aPS
3.2. Axial Stretching of Films with CC PPO/Toluene Phases
3.2.1. Axial Stretching of CC PPO/Toluene Films
3.2.2. Axial Stretching of PPO/aPS Films with CC PPO/Toluene Phase
3.3. Axial Stretching of Films with CC PPO/Mesitylene Phases
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aguilar-Vega, M.; Paul, D.R. Gas transport properties of polyphenylene ethers. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1577–1589. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T.; Lamarche, G.; Kim, H.J. The morphology characterization and performance of dense PPO membranes for gas separation. J. Membr. Sci. 1997, 135, 211–223. [Google Scholar] [CrossRef]
- Xu, T.; Wu, D.; Wu, L. Poly (2, 6-dimethyl-1, 4-phenylene oxide) (PPO)—A versatile starting polymer for proton conductive membranes (PCMs). Prog. Polym. Sci. 2008, 33, 894–915. [Google Scholar] [CrossRef]
- Xue, J.; Liu, X.; Zhang, J.; Yin, Y.; Guiver, M.D. Poly (phenylene oxide) s incorporating N-spirocyclic quaternary ammonium cation/cation strings for anion exchange membranes. J. Membr. Sci. 2020, 595, 117507. [Google Scholar] [CrossRef]
- De Rosa, C.; Guerra, G.; Petraccone, V.; Pirozzi, B. Crystal structure of the emptied clathrate form (δe form) of syndiotactic polystyrene. Macromolecules 1997, 30, 4147–4152. [Google Scholar] [CrossRef]
- Daniel, C.; Alfano, D.; Venditto, V.; Cardea, S.; Reverchon, E.; Larobina, D.; Mensitieri, G.; Guerra, G. Aerogels with microporous crystalline host phase. Adv. Mater. 2005, 17, 1515–1518. [Google Scholar] [CrossRef]
- Kaneko, F.; Uda, Y.; Tanigaki, N.; Kawaguchi, T. The First example of a polymer-crystal–organic-dye composite material: The clathrate phase of syndiotactic polystyrene with azulene. Adv. Mater. 2005, 17, 1846–1850. [Google Scholar]
- Petraccone, V.; De Ballesteros, O.R.; Tarallo, O.; Rizzo, P.; Guerra, G. Nanoporous polymer crystals with cavities and channels. Chem. Mater. 2008, 20, 3663–3668. [Google Scholar] [CrossRef]
- Gowd, E.B.; Tashiro, K.; Ramesh, C. Structural phase transitions of syndiotactic polystyrene. Progr. Polym. Sci. 2009, 34, 280–315. [Google Scholar] [CrossRef]
- Acocella, M.R.; Rizzo, P.; Daniel, C.; Tarallo, O.; Guerra, G. Nanoporous triclinic δ modification of syndiotactic polystyrene. Polymer 2015, 63, 230–236. [Google Scholar] [CrossRef]
- Schiavone, M.M.; Tarallo, O.; Di Girolamo, R.; Caporaso, L.; Appavou, M.-S.; Revay, Z.; Radulescu, A. Structure and morphology of model polymer electrolyte membranes based on sulfonated syndiotactic-polystyrene in the δ co-crystalline phase resolved by small-angle neutron scattering. Solid State Ion. 2018, 320, 392–406. [Google Scholar] [CrossRef]
- Naga, N.; Sakurai, T.; Hashimoto, T.; Noguchi, K. Crystalline structure and phase transition of syndiotactic styrene-based copolymers. Polym. Int. 2019, 68, 71–78. [Google Scholar] [CrossRef]
- Daniel, C.; Longo, S.; Fasano, G.; Vitillo, J.G.; Guerra, G. Nanoporous crystalline phases of poly (2, 6-Dimethyl-1, 4-phenylene) oxide. Chem. Mater. 2011, 23, 3195–3200. [Google Scholar] [CrossRef]
- Galizia, M.; Daniel, C.; Fasano, G.; Guerra, G.; Mensitieri, G. Gas sorption and diffusion in amorphous and semicrystalline nanoporous poly (2, 6-dimethyl-1, 4-phenylene) oxide. Macromolecules 2012, 45, 3604–3615. [Google Scholar] [CrossRef]
- Galizia, M.; Daniel, C.; Guerra, G.; Mensitieri, G. Solubility and diffusivity of low molecular weight compounds in semi-crystalline poly-(2, 6-dimethyl-1, 4-phenylene) oxide: The role of the crystalline phase. J. Membr. Sci. 2013, 443, 100–106. [Google Scholar] [CrossRef]
- Daniel, C.; Zhovner, D.; Guerra, G. Thermal stability of nanoporous crystalline and amorphous phases of poly (2, 6-dimethyl-1, 4-phenylene) oxide. Macromolecules 2013, 46, 449–454. [Google Scholar] [CrossRef]
- Daniel, C.; Pellegrino, M.; Venditto, V.; Aurucci, S.; Guerra, G. Nanoporous-crystalline poly (2, 6-dimethyl-1, 4-phenylene) oxide (PPO) Aerogels. Polymer 2016, 105, 96–103. [Google Scholar] [CrossRef]
- Lova, P.; Bastianini, C.; Giusto, P.; Patrini, M.; Rizzo, P.; Guerra, G.; Iodice, M.; Soci, C.; Comoretto, D. Label-free vapor selectivity in poly (p-phenylene oxide) photonic crystal sensors. ACS Appl. Mater. Interfaces 2016, 8, 31941–31950. [Google Scholar] [CrossRef] [Green Version]
- Nagendra, B.; Cozzolino, A.; Daniel, C.; Rizzo, P.; Guerra, G.; Auriemma, F.; De Rosa, C.; DAlterio, M.; Tarallo, O.; Nuzzo, A. Two nanoporous crystalline forms of poly (2, 6-dimethyl-1, 4-phenylene) oxide and related Co-crystalline forms. Macromolecules 2019, 52, 9646–9656. [Google Scholar] [CrossRef]
- Daniel, C.; Antico, P.; Yamaguchi, H.; Kogure, M.; Guerra, G. Microporous-crystalline microfibers by eco-friendly guests: An efficient tool for sorption of volatile organic pollutants. Micropor. Mesopor. Mat. 2016, 232, 205–210. [Google Scholar] [CrossRef]
- Daniel, C.; Antico, P.; Guerra, G. Etched fibers of syndiotactic polystyrene with nanoporous-crystalline phases. Macromolecules 2018, 51, 6138–6148. [Google Scholar] [CrossRef]
- Gui, H.; Zhang, T.; Guo, Q. Nanofibrous, emulsion-templated syndiotactic polystyrenes with superhydrophobicity for oil spill cleanup. ACS Appl. Mater. Interfaces 2019, 11, 36063–36072. [Google Scholar] [CrossRef] [PubMed]
- Pilla, P.; Cusano, A.; Cutolo, A.; Giordano, M.; Mensitieri, G.; Rizzo, P.; Sanguigno, L.; Venditto, V.; Guerra, G. Molecular sensing by nanoporous crystalline polymers. Sensors 2009, 9, 9816–9857. [Google Scholar] [CrossRef] [PubMed]
- Larobina, D.; Sanguigno, L.; Venditto, V.; Guerra, G.; Mensitieri, G. Gas sorption and transport in syndiotactic polystyrene with nanoporous crystalline phase. Polymer 2004, 45, 429–436. [Google Scholar] [CrossRef]
- Rizzo, P.; Cozzolino, A.; Albunia, A.R.; Giuffre, A.M.; Sicari, V.; Di Maio, L.; Daniel, C.; Venditto, V.; Galimberti, M.; Mensitieri, G.; et al. Packaging technology for improving shelf-life of fruits based on a nanoporous-crystalline polymer. J. Appl. Polym. Sci. 2018, 135, 46256. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P.; Longo, S.; Venditto, V.; Guerra, G. N-doped TiO2/s-PS aerogels for photocatalytic degradation of organic dyes in wastewater under visible light irradiation. J. Chem. Tech. Biotech. 2014, 89, 1175–1181. [Google Scholar] [CrossRef]
- Noschese, A.; Buonerba, A.; Canton, P.; Milione, S.; Capacchione, C.; Grassi, A. Highly efficient and selective reduction of nitroarenes into anilines catalyzed by gold nanoparticles incarcerated in a nanoporous polymer matrix: Role of the polymeric support and insight into the reaction mechanism. J. Catal. 2016, 340, 30–40. [Google Scholar] [CrossRef]
- Rizzo, P.; Cozzolino, A.; Guerra, G. Chemical stabilization of hexanal molecules by inclusion as guests of nanoporous-crystalline syndiotactic polystyrene crystals. Macromolecules 2019, 52, 2255–2264. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Ianniello, G.; Rufolo, C.; Guerra, G. Syndiotactic polystyrene films with a cocrystalline phase including carvacrol guest molecules. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 657–665. [Google Scholar] [CrossRef]
- Stegmaier, P.; De Girolamo Del Mauro, A.; Venditto, V.; Guerra, G. Optical recording materials based on photoisomerization of guest molecules of a polymeric crystalline host phase. Adv. Mater. 2005, 17, 1166–1168. [Google Scholar] [CrossRef]
- Itagaki, H.; Sago, T.; Uematsu, M.; Yoshioka, G.; Correa, A.; Venditto, V.; Guerra, G. Guest orientation in uniplanar-axial polymer host films and in co-crystal unit-cell, determined by angular distributions of polarized guest fluorescence. Macromolecules 2008, 41, 9156–9164. [Google Scholar] [CrossRef]
- Rizzo, P.; Abbate, S.; Longhi, G.; Guerra, G. Circularly polarized luminescence of syndiotactic polystyrene. Opt. Mater. 2017, 73, 595–601. [Google Scholar] [CrossRef]
- Albunia, A.R.; D’Aniello, C.; Guerra, G.; Gatteschi, D.; Mannini, M.; Sorace, L. Ordering magnetic molecules within nanoporous crystalline polymers. Chem. Mater. 2009, 21, 4750–4752. [Google Scholar] [CrossRef]
- Daniel, C.; Rufolo, C.; Bobba, F.; Scarfato, A.; Cucolo, A.M.; Guerra, G. Ferroelectric co-crystalline polymers. J. Mater. Chem. 2011, 21, 19074–19079. [Google Scholar] [CrossRef]
- Kobayashi, H.; Akazawa, S.; Urakawa, O.; Kaneko, F.; Inoue, T. Anisotropic dynamics of benzonitrile confined in δ and ε clathrate phases of syndiotactic polystyrene. Macromolecules 2018, 51, 8611–8619. [Google Scholar] [CrossRef]
- Guerra, G.; Daniel, C.; Rizzo, P.; Tarallo, O. Advanced materials based on polymer co-crystalline forms. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 305–322. [Google Scholar] [CrossRef]
- Uda, Y.; Kaneko, F.; Kawaguchi, T. Guest exchange with n-alkanes and host-guest interactions in the clathrate phase of syndiotactic polystyrene. Macromolecules 2005, 38, 3320–3326. [Google Scholar] [CrossRef]
- Albunia, A.R.; Di Masi, S.; Rizzo, P.; Milano, G.; Musto, P.; Guerra, G. Chlorinated guest orientation and mobility in clathrate structures formed with syndiotactic polystyrene. Macromolecules 2003, 36, 8695–8703. [Google Scholar] [CrossRef]
- Albunia, A.R.; Milano, G.; Venditto, V.; Guerra, G. A clear-cut experimental method to discriminate between in-plane and out-of-plane molecular transition moments. J. Am. Chem. Soc. 2005, 127, 13114–13115. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Coppola, M.; De Pascale, M.; Guerra, G. Azobenzene isomerization in polymer co-crystalline phases. Polymer 2012, 53, 2727–2735. [Google Scholar] [CrossRef]
- Albunia, A.R.; Guerra, G. Spectroscopic investigation of guest-guest interactions in the nanoporous-crystalline δ and ε forms of syndiotactic polystyrene. J. Phys. Chem. C 2014, 118, 11774–11783. [Google Scholar] [CrossRef]
- Golla, M.; Nagendra, B.; Rizzo, P.; Daniel, C.; De Ballesteros, O.R.; Guerra, G. Polymorphism of poly(2, 6-dimethyl-1, 4-phenylene) oxide in axially stretched films. Macromolecules 2020, 53, 2287–2294. [Google Scholar] [CrossRef]
- Golla, M.; Nagendra, B.; Fierro, F.; Rizzo, P.; Daniel, C.; Guerra, G. Axially oriented nanoporous crystalline phases of poly (2, 6-dimethyl-1, 4-phenylene)oxide. ACS Appl. Polym. Mater. 2020, 2, 3518–3524. [Google Scholar] [CrossRef]
- Daniel, C.; Galdi, N.; Montefusco, T.; Guerra, G. Syndiotactic polystyrene clathrates with polar guest molecules. Chem. Mater. 2007, 19, 3302–3308. [Google Scholar] [CrossRef]
- Tarallo, O.; Schiavone, M.M.; Petraccone, V.; Daniel, C.; Rizzo, P.; Guerra, G. Channel clathrate of syndiotactic polystyrene with p-nitroaniline. Macromolecules 2010, 43, 1455–1466. [Google Scholar] [CrossRef]
- Barrales-Rienda, M.; Fatou, J.M.G. Single crystals of poly (2, 6-dimethyl-1, 4-phenylene) oxide. Kolloid-Z. Z. Polym. 1971, 244, 317–323. [Google Scholar] [CrossRef]
- Horikiri, S. Single crystals of poly (2, 6-dimethylphenylene Oxide). J. Polym. Sci. Part A-2 Polym. Phys. 1972, 10, 1167–1170. [Google Scholar] [CrossRef]
- Hurek, J.; Turska, E. X-ray Studies of Crystallization of Poly (2,6-dimethyl-1,4-phenylene oxide) on Swelling in Certain Systems. Acta Polym. 1984, 35, 201–207. [Google Scholar] [CrossRef]
- Tarallo, O.; Petraccone, V.; Daniel, C.; Fasano, G.; Rizzo, P.; Guerra, G. A chiral co-crystalline form of poly (2, 6-dimethyl-1, 4-phenylene) oxide (PPO). J. Mater. Chem. 2012, 22, 11672–11680. [Google Scholar] [CrossRef]
- Tasumi, M.; Urano, T.; Nakata, M. Some thoughts on the vibrational modes of toluene as a typical monosubstituted benzene. J. Mol. Struct. 1986, 146, 383–396. [Google Scholar] [CrossRef]
- Nagendra, B. Unpublished data.
- Rizzo, P.; Gallo, C.; Vitale, V.; Tarallo, O.; Guerra, G. Nanoporous-crystalline films of PPO with parallel and perpendicular polymer chain orientations. Polymer 2019, 167, 193–201. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golla, M.; Nagendra, B.; Daniel, C.; Rizzo, P.; Guerra, G. Axial Orientation of Co-Crystalline Phases of Poly(2,6-Dimethyl-1,4-Phenylene)Oxide Films. Polymers 2020, 12, 2394. https://doi.org/10.3390/polym12102394
Golla M, Nagendra B, Daniel C, Rizzo P, Guerra G. Axial Orientation of Co-Crystalline Phases of Poly(2,6-Dimethyl-1,4-Phenylene)Oxide Films. Polymers. 2020; 12(10):2394. https://doi.org/10.3390/polym12102394
Chicago/Turabian StyleGolla, Manohar, Baku Nagendra, Christophe Daniel, Paola Rizzo, and Gaetano Guerra. 2020. "Axial Orientation of Co-Crystalline Phases of Poly(2,6-Dimethyl-1,4-Phenylene)Oxide Films" Polymers 12, no. 10: 2394. https://doi.org/10.3390/polym12102394
APA StyleGolla, M., Nagendra, B., Daniel, C., Rizzo, P., & Guerra, G. (2020). Axial Orientation of Co-Crystalline Phases of Poly(2,6-Dimethyl-1,4-Phenylene)Oxide Films. Polymers, 12(10), 2394. https://doi.org/10.3390/polym12102394