1. Introduction
A microscopic integrated device (MID) is an injection-moulded thermoplastic part with electronic circuits directly integrated on a polymeric component. It offers material, weight and cost savings by the elimination of connectors between separate printed circuit boards (PCBs), the shortening of the process chain and integration of contact surfaces, e.g., for switches, sensors and antennas [
1]. MIDs have great potential in automotive, aviation, lighting, computing or even medical sectors where emerging innovation requires the increase of the number of the electronic components in a device. The MID approach requires a combination of various technologies to fabricate a product from different materials. A final product integrates multiple mechanical and electrical functionalities.
The main technological problem of MIDs is that of producing electrical circuit traces on plastic parts. Standard known techniques such as photolithography cannot be applied conventionally since the parts generally have complex 3D geometrical shapes. There are several methods of the laser-induced local metal deposition on polymers than can be used for circuit trace fabrication: metal nano-ink printing [
2,
3], ink paste layer sintering [
4,
5], laser-induced selective activation [
6] and laser direct structuring (LDS) [
7]. Injection printing uses liquid ink, usually made from silver nanoparticles. The thermal or piezoelectric method can be applied for droplet ejection. Presently, almost all industrial machines use piezoelectric heads [
2]. There are several methods for ink paste sintering, but all of them have many similar procedures. A nonconductive ink solution based on metal compounds is reduced to metal atoms by laser irradiation. Kang et al. [
4] made ink paste by dispersing copper oxide (CuO) particles into a reducing agent solution. The solution consisting of polyvinylpyrrolidone (PVP
Mw 10,000, Aldrich, 13 wt %) and ethylene glycol (Aldrich, 27 wt %) was mixed with nanoparticles by an ultrasonic wave. Finally, the CuO NP solution with a viscosity of 5000 cps was achieved [
4]. The solution was deposited on polyimide (PI) by a spin-coating technique. Ytterbium-doped fibre laser was used for irradiation of the deposited layer. Both pulsed nanosecond and CW lasers were applied. Photon energy of the irradiating laser had an energy high enough to break Cu–O bonds. After bond breaking, a reduction of Cu ions by ethylene glycol took place. The results were checked by energy-dispersive X-ray spectroscopy (EDS), and it was found that 90% of Cu ions could be reduced by pulse laser irradiation.
In a study carried out by Chen et al. [
5], PI films were treated by KOH solution. As a result, PI became hydrolysed, and potassium polyamine was generated. Later, the specimen was immersed in the AgNO
3 solution, allowing potassium ions to be exchange by the Ag ions. Laser writing was performed by Nd:YAG laser nanosecond laser with the IV harmonic wavelength. After laser irradiation, the Ag ions were reduced to Ag
0 [
5]. The disadvantages of both processes are very similar concerning the difficulties of thin layer deposition. In the case of the complex 3D surface, it becomes almost impossible.
Another technological problem is very low laser writing speed in the hundreds of microns/s during the reduction process. Zhang et al. presented a technology [
6] for selective copper plating on laser-modified areas in a water environment, followed by chemical palladium activation and electroless plating. Firstly, the polymeric specimen—polycarbonate—was immersed in distilled water. The fundamental radiation of an Nd:YAG nanosecond laser was used to modify the polymer surface in the water. After the treatment, a sample was activated in palladium colloidal solution for several minutes. The rinsing after activation with palladium was performed in the distilled water. The last step, electroless copper plating, was applied for deposition of a conductive circuit. The selective plating process was also been attempted by performing laser activation in the air. However, no selective plating was achieved. The last two methods utilise chemical metal deposition in addition to laser treatment. All of them except LDS face difficulties when they are applied to three-dimensional parts.
LDS is the method of using precursors mixed in a polymer matrix [
7]. These precursor additives are activated during the laser writing process by converting them into a catalyst for electroless deposition of the metal. Thus, the laser-treated area can be selectively plated [
7]. LDS is a state-of-the-art method and the most commercialised process for MID production. There are a few commercial materials for LDS available on the market. However, most of the LDS polymers are based on expensive metal–organic fillers, such as palladium-based metal–organic compounds or microparticles of copper oxide spinel crystal [
7], which increases the price of the raw material several times (for example, a cost ratio is 1:4 in the case of comparing pure PC/ABS to one with LDS additives) [
8]. Therefore, the cost of the material limits the further expansion of the technology to the automotive and consumer goods sectors. In addition, a high required concentration of metal–organic additives reduces mechanical properties of a moulded part—moreover, metal-based additives in the polymer matrix shield electromagnetic radiation and limit MID application in the gigahertz frequency range [
9,
10].
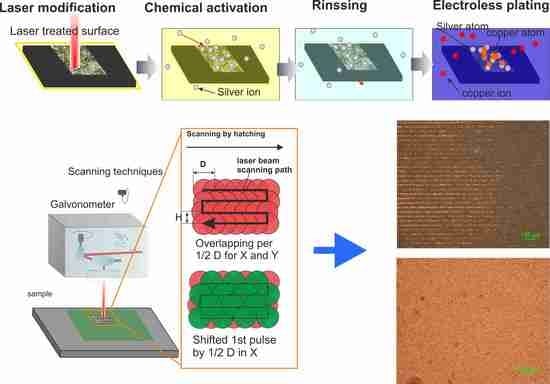
One of the most promising technologies to overcome the aforementioned challenges is the selective surface activation induced by laser (SSAIL) [
11,
12]. The process includes laser modification of the polymer surface with a short-pulse laser, chemical activation of laser-modified areas and electroless metal deposition on the locally activated surface. An essential parameter for industrial application is the processing speed. The SSAIL technology consists of three chemical steps of dipping into liquids and one laser treatment step. From an industrial point of view, the chemical process could be easily upscaled by increasing the size of baths, allowing a large number of parts to be processed at once [
13]. The laser process should be fast as laser time is expensive [
8]. SSAIL technology offers high laser modification speed for high-performance polymeric materials like PolyEther Ether Ketone (PEEK) and Liquid Crystal Polymer (LCP) [
12]. However, the rate of laser processing of widely used engineering plastics such as PC, ABS or PC/ABS is much lower. For example, for one of the most popular automotive engineering plastics, ABS, the laser writing speed of the SSAIL process is only 0.5 m/s [
14]. The higher speed is limited by the unwanted thermal effects that can be caused by heat accumulation [
15]. Thermal effects can result in the reduced adhesion to a substrate or lower spatial resolution of plating, or they can lead to areas not being covered by metal at all. There are several known solutions to the problem, such as cooling by gas agitation [
16] or by water [
17]. However, these methods are applied and then processing is carried out through a nozzle while the sample is positioned only by the mechanical axis. In the SSAIL process, fast laser processing is achieved by galvanometric scanning, and implementation of these cooling techniques would be complicated or even impossible. Another option for the elimination of thermal effects is to apply an advanced laser writing technique. J. Martan et al. have shown a special shifted laser writing method for high-speed texturing of metals [
18,
19] with a polygon scanner. Their approach permitted the processing speed to be significantly increased.
In this work, the laser writing technique at the maximum scanning speed using interlacing of pulses and lines with overlapping on the next scanning runs over the same area was implemented for the SSAIL process on PC/ABS polymer substrate. The potential benefits of the new technique were initially checked by finite element method (FEM) modelling of the heat equation after laser beam absorption in PC/ABS. The experiments were carried out using various laser processing parameters for the advanced laser writing method. The quality of the final plated copper layer was checked to determine the impact of the laser scanning approach. Optical and SEM microscopy, electrical sheet resistance and energy-dispersive X-ray spectroscopy (EDS) were applied to check the quality of the deposited copper layer.
3. Results
Initially, the interlacing pulse technique was applied using X1/2D, X1D and X3/2D types of scanning with varying average laser power: 5, 10, 15, 20 and 25 W. Conventional scanning was applied as a reference. The maximum laser writing speed of 4.8 m/s was used at the 400 kHz pulse repetition rate. Optical microscope images, 1.5 × 1.5 mm
2 in size, of surface areas after the complete SSAIL (final copper deposition) procedures for different laser writing techniques are shown in
Figure 5.
Copper colour areas are clearly seen in
Figure 5. The smooth plating over the entire treated surface was achieved, even after applying X1/2D type of scanning with only two scanning runs, with 5 W of average laser power. Increasing the distance between pulses in the X direction and the number of scanning runs led to a broader window of processing parameters for higher laser power and irradiation doses. This result clearly shows that laser processing speed for SSAIL process is limited by heat accumulation.
SSAIL, a selective copper deposition procedure, was applied for interlacing pulse technique by increasing the distance between pulses in the Y direction, in other words, increasing the distance between parallel scanned lines, as explained in
Figure 2. The Y1/2D, Y1D, Y3/2D types of laser writing were performed by scanning. When increasing the distance between pulses in the X direction, for example, by applying X1/2D writing, the processing time is doubled because the laser beam needs to travel over the whole scanned line in both scans. The same in the Y direction (perpendicular to scanned lines) does not increase processing time, since laser beam can simply skip a line within the short jump to the next line. The combination of both processes—increasing the distance between pulses in both directions—was tested as well. Optical images of deposited copper after applying different types of shifted pulse method are presented in
Figure 6. Two different average laser power settings were used: 25 and 15 W. The laser power was chosen as shown in
Figure 5, typically failing to obtain the plating if the conventional or the interlacing pulses in the X direction techniques were used. Applying only interlacing pulses in the Y direction did not allow continuous plating over the surface to be achieved, as shown in
Figure 6, but the combination of both processes provided a fully covered surface for both values of average laser power. For higher average power (25 W), the combined process ensured better results, as shown in
Figure 6b, as the Y1D × X3/2D area appears to be continuously covered.
Electrical sheet resistance measurements were carried out to quantitatively evaluate the deposited copper layer. The four-probe technique was used for the resistance tests. Electrical measurements can indicate the quality of the plated surface even for optically undetectable problems, such as copper purity, or tiny areas of underplating. Results of the sheet resistance measurements are presented in
Figure 7; they were obtained on the areas depicted in
Figure 5 and
Figure 6. It is clear from
Figure 7a that the sheet resistance decreased after applying interlacing pulse scanning procedure in the X-direction. A drastic decrease in the sheet resistance indicates that a continuous copper layer is formed. For higher average laser power, a greater distance between pulses in the X direction is needed to achieve low sheet resistance values. In the case of 25 W, even after X3/2D type of scanning, the resistance remained high, showing that there was no continuous copper layer. After applying a combination of increasing the distance between pulses on the first run in both X and Y directions, it was possible to deposit a copper layer, even when the high laser power (25 W) was used, as demonstrated in
Figure 7b.
However, the application of interlacing pulses in the Y direction even after low power of irradiation did not guarantee the continuously plated surface when 4.8 m/s scanning speed was applied.
Figure 8 shows the optical microscope image of deposited copper after application of 5 W average laser power and Y3/2D. In the picture, visible dark lines indicate that coverage is not full along the plated stripe.
Further samples were treated with at a lower speed of laser beam writing, 0.8–4.8 m/s for the X1/2D and Y1/2D, Y1D, Y3/2D scanning types, applying the 5 W average laser power and 400 kHz pulse repetition rate. The results were compared with the conventional scanning with several best possible regimes of laser irradiation and writing parameters [
14]. The sheet resistance dependence on laser processing speed is presented in
Figure 9. The results are described not by laser writing speed but by the overall processing speed instead, including that scanning by X1/2D requires two scans when compared with the conventional scanning with the same writing speed. The fully covered copper layer has the resistance of less than 1 Ω/sq, as shown in
Figure 9 by the green dashed line. All values below this line were considered as continuously plated—the positive experimental result. The fastest processing speed for the conventional scanning technique was 1 m/s, and the value of resistance was very close to the boundary line. The processing speed could be increased if the Y1/2D scanning technique was applied. However, no improvement was observed when the Y1D technique was used.
The most favourable results were achieved for the X1/2D scanning technique. The process works with the highest 4.8 m/s laser writing speed and provides a 2.4 m/s processing speed. The best copper plating quality was achieved for the X1/2D scanning technique as well since the low sheet resistance of plated copper was measured. Lower sheet resistance in the case of conventional scanning at a low pulse repetition rate like 10 or 20 kHz could be caused by a thicker layer of copper, as the plating was carried longer [
14].
Another critical parameter is plating selectivity.
Figure 10 shows the plated lines with X1/2D, X1D and X3/2D scanning techniques using 5 W average laser power.
From
Figure 10, it can be easily seen that the plated lines where X1D and X3/2D writing was applied have smoother edges. This could be a consequence of the mitigation of heat accumulation by interlaced scanning.
Scanning electron microscope imaging was used to analyse the surface morphology. Laser-treated surfaces subjected to the interlacing pulses in the X direction writing technique were analysed and compared. In some cases, a significant decrease of surface melting was observed when the increase of the distance between pulses in the X direction was applied for the first scanning run.
Figure 11 presents examples of surface morphology written by increasing the distance between pulses in the X direction and using conventional scanning.
Figure 11a shows a surface irradiated with 20 W average laser power, and
Figure 11b shows a surface irradiated with 15 W average laser power. When applying a greater distance between pulses on the first scanning run, the surface’s structure is entirely different—a submicron structure is formed. Meanwhile, after the conventional writing technique, the surface is remelted, and the structure has micrometre-sized features. Nevertheless, the significant reduction of thermal effects, like eliminating remelting of surface, in the modified surface in
Figure 11a,b where X3/2D and X1D writing is applied is not suitable for copper deposition using SSAIL.
In
Figure 12a, the surface processed with 15 W average laser power and scanned with X1D is compared with the surface treated with the same laser parameters but applying X3/2D writing technique. In
Figure 12b, the surface processed with 10 W average laser power and scanned with X1/2D is compared with the surface processed with the same laser parameters but applying X1D writing. In both pictures, no significant difference can be seen as resulting after increasing the distance between pulses. However, the surface is not active for SSAIL, and no copper can be deposited after 15 W average laser power with X1D scanning treatment. The same could be said about the surface after 10 W laser power with X1/2D scanning treatment. However, after increasing the distance between pulses in the scanning direction by one-half of the beam diameter for both cases, the surface becomes active for SSAIL process. In other words, the chemical activation can take place in the irradiated areas. This result could be explained by reduced accumulation of temperature, although no morphological structure changes on the surface are observed. This result proves that SSAIL process is caused not only by the morphological structures on the surface after the laser modification.
All experimental investigations show that thermal accumulation is a limiting factor for fast laser writing in the SSAIL process. However, the consequences of the heat accumulation are not fully understood yet, since SEM pictures show that morphology can look almost identical for the active and non-active surfaces for electroless deposition using the SSAIL technique. Therefore, we assume that differences in surface activation could come from chemical modifications in the laser-treated areas.
EDS was applied to measure the ratio of carbon to oxygen atomic concentration on the surface of polymer irradiated with the laser. Laser processing regimes indicated in
Figure 5 were used for the treatment of samples. The C/O ratio dependence on the increase of distance between pulses for various average laser powers is presented in
Figure 13. The reference ratio of atomic C/O concentration for the non-processed surface was 3.47. After the laser treatment, the C/O ratio increased, showing a decrease of oxygen content on the surface. Increase in the distance between pulses in the scanning direction resulted in a lower oxygen concentration when 5, 10 or 15 W average laser power was used. EDS measurements describe the relation between C/O ratio and activity of a surface for electroless copper deposition using the SSAIL process. Laser processing parameters when the surface of PC/ABS was activated for SSAIL are marked in orange colour in
Figure 13. All surfaces which were active for SSAIL after the laser treatment had a C/O ratio above 5.75. This result shows that the decrease in oxygen content on the PC/ABS surface plays a crucial role in laser excitation process for chemical activation with silver nitrate.
There are some works that present the decrease in oxygen content on the polymer surface after laser irradiation with excimer lasers [
22]. The excimer laser works in ultraviolet wavelength range of approximately 340–250 nm, while our research was performed with the 532 nm wavelength. Using an excimer laser, carbonyl and hydroxy groups (which contain oxygen) on polymer surface can be destroyed photochemically. Photon energy at the 532 nm wavelength is not high enough to break chemical bonds of hydroxy or oxygenated chemical groups. However, pulse duration (used in this work) is more than 1000 times shorter than that of excimer lasers, and nonlinear multiphoton absorption could be dominant, enabling chemical bonds to be broken with lower photon energy. Wu et al. [
23] reported oxygenated chemical groups that were naturally formed on a polycarbonate surface in ambient air. The content of PC in PC/ABS blend is significantly higher: 70–90% by weight. Therefore, much more PC material interacts with laser irradiation than PC/ABS blend.
Figure 14a shows the surface chemical condition of PC in ambient air. Carbonyl C=O groups are localised on the PC surface, and ester groups are also located very close to the surface. Polycarbonates can also contain some amounts of carboxylic groups. It is well known that during the reduction of esters or carbocyclic acids, ketones and aldehydes are formed, respectively. In both cases, the loss in oxygen amount was observed (see
Figure 14).
As the decrease of oxygen content on the surface was observed after the laser treatment for surface excitation, we can conclude that the picosecond laser pulse nonlinearly breaks bonds of carbonyl and ester groups directly on the surface or below it, and the reduction process takes place. As a result, the formation of the aldehydic groups on the surface could be expected, as shown in
Figure 15b.
After the laser treatment, a sample was immersed in AgNO3 activation solution, and localised aldehydic groups on laser-treated areas reacted with Ag+ very easily and actively. As a result, metallic Ag was produced, which acted later as a catalyst for the electroless copper deposition process.
The suggested interpretation explains the selectiveness of plating for the SSAIL process. Moreover, it explains the different metal deposition behaviours on the surfaces that look almost identical regarding the surface morphology (
Figure 12).
SSAIL technology is orientated to practical industrial application. Therefore, the time for laser treatment of the real 3D items was estimated.
Figure 16a presents a 3D MID part that was processed using pulse interlacing laser scanning method. The X1/2D writing technique was used for the machining of the item. In this example, the laser processing time for the conventional scanning method (1 min) was reduced to 25 s for a single item.