Degradation of Polylactic Acid Using Sub-Critical Water for Compost
Abstract
:1. Introduction
- Molecular weight reduction;
- Restriction of production of small molecules, which are organic acids such as lactic acid and lactic acid dimer, because they contribute to soil acidification.
2. Materials and Methods
2.1. Materials
2.2. Batch-Type Reactor
2.3. Analysis of yields
2.4. Analysis of Kinetics
2.5. Measurement of Soil pH
3. Results
3.1. Degradation of PLA Chains
3.2. Crystallization of PLA
3.3. WSC Ratio of PLA
3.4. Yield of Water-Soluble Components
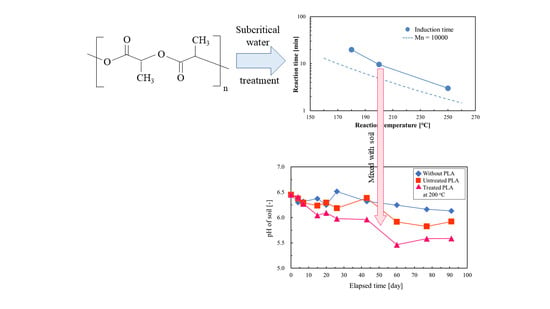
3.5. pH of Soil Mixed with PLA
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Avérous, L. Monomers, Polymers and Composites from Renewable Resources; Elsevier: Oxford, UK; Amsterdam, The Netherlands, 2008; pp. 433–450. [Google Scholar]
- Di Lorenzo, M.L.; Androsch, R. Industrial Applications of Poly(Lactic Acid); Springer: Berlin, Germany, 2018. [Google Scholar]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [Green Version]
- Karamanlioglu, M.; Robson, G.D. The influence of biotic and abiotic factors on the rate of degradation of poly(lactic) acid (PLA) coupons buried in compost and soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; Wadsworth, L.C.; Anunciado, M.B.; English, M.E.; Bandopadhyay, S.; Schaeffer, S.M.; Debruyn, J.M.; Miles, C.A.; et al. In situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci. Total. Environ. 2020, 727, 138668. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Houlden, A.; Robson, G.D. Isolation and characterisation of fungal communities associated with degradation and growth on the surface of poly(lactic) acid (PLA) in soil and compost. Int. Biodeterior. Biodegrad. 2014, 95, 301–310. [Google Scholar] [CrossRef]
- Hottle, T.; Agüero, M.L.; Bilec, M.M.; Landis, A.E. Alkaline Amendment for the Enhancement of Compost Degradation for Polylactic Acid Biopolymer Products. Compos. Sci. Util. 2016, 24, 159–173. [Google Scholar] [CrossRef]
- Wadsworth, L.C.; Hayes, D.G.; Wszelaki, A.L.; Washington, T.L.; Martin, J.; Lee, J.; Raley, R.; Pannell, C.T.; Dharmalingam, S.; Miles, C.; et al. Evaluation of Degradable Spun-Melt 100% Polylactic Acid Nonwoven Mulch Materials in a Greenhouse Environment. J. Eng. Fibers Fabr. 2013, 8, 526–536. [Google Scholar] [CrossRef]
- Tisserat, B.; Finkenstadt, V.L. Degradation of Poly(l-Lactic Acid) and Bio-Composites by Alkaline Medium Under Various Temperatures. J. Polym. Environ. 2011, 19, 766–775. [Google Scholar] [CrossRef]
- Tavanaie, M.A. Melt Recycling of Poly(lactic Acid) Plastic Wastes to Produce Biodegradable Fibers. Polym. Technol. Eng. 2014, 53, 742–751. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Biodegradable electrospun bionanocomposite fibers based on plasticized PLA–PHB blends reinforced with cellulose nanocrystals. Ind. Crop. Prod. 2016, 93, 290–301. [Google Scholar] [CrossRef]
- Nascimento, L.; Gámez-Pérez, J.; Santana, O.O.; Velasco, J.I.; Maspoch, M.L.; Franco-Urquiza, E.A. Effect of the Recycling and Annealing on the Mechanical and Fracture Properties of Poly(Lactic Acid). J. Polym. Environ. 2010, 18, 654–660. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M. Kinetics of the thermal decomposition of processed poly(lactic acid). Polym. Degrad. Stab. 2010, 95, 2508–2514. [Google Scholar] [CrossRef]
- Auras, R.; Lim, L.-T.; Selke, S.E.M.; Tsuji, H. Poly(Lactic Acid) Synthesis, Structures, Properties, Processing and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 343–382, 401–410. [Google Scholar]
- Song, X.; Bian, Z.; Hui, Y.; Wang, H.; Liu, F.; Yu, S. Zn-Acetate-Containing ionic liquid as highly active catalyst for fast and mild methanolysis of Poly(lactic acid). Polym. Degrad. Stab. 2019, 168, 2760–2764. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, R.; Song, X.; Yu, S.; Ge, X. Lewis Acidic Ionic Liquid [Bmim]FeCl4 as a High Efficient Catalyst for Methanolysis of Poly(lactic acid). Catal. Lett. 2017, 147, 2298–2305. [Google Scholar] [CrossRef]
- Petrus, R.; Bykowski, D.; Sobota, P. Solvothermal Alcoholysis Routes for Recycling Polylactide Waste as Lactic Acid Esters. ACS Catal. 2016, 6, 5222–5235. [Google Scholar] [CrossRef]
- Plichta, A.; Lisowska, P.; Kundys, A.; Zychewicz, A.; Dębowski, M.; Florjańczyk, Z. Chemical recycling of poly(lactic acid) via controlled degradation with protic(macro)molecules. Polym. Degrad. Stab. 2014, 108, 288–296. [Google Scholar] [CrossRef]
- Román-Ramírez, L.A.; McKeown, P.; Jones, M.D.; Wood, J. Kinetics of Methyl Lactate Formation from the Transesterification of Polylactic Acid Catalyzed by Zn(II) Complexes. ACS Omega 2020, 5, 5556–5564. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M.; Grzybowski, Ł.; Czupryński, B. Glycerolysis of Poly(lactic acid) as a Way to Extend the “Life Cycle” of This Material. Polymers 2019, 11, 1963. [Google Scholar] [CrossRef] [Green Version]
- Román-Ramírez, L.A.; McKeown, P.; Shah, C.; Abraham, J.; Jones, M.D.; Wood, J. Chemical Degradation of End-of-Life Poly(lactic acid) into Methyl Lactate by a Zn(II) Complex. Ind. Eng. Chem. Res. 2020, 59, 11149–11156. [Google Scholar] [CrossRef]
- Terrade, F.G.; Van Krieken, J.; Verkuijl, B.J.V.; Bouwman, E. Catalytic Cracking of Lactide and Poly(Lactic Acid) to Acrylic Acid at Low Temperatures. ChemSusChem 2017, 10, 1904–1908. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Dolan, K.; Selke, S.; Holmes, D.; Rubino, M.; Soto-Valdez, H. Chemical recycling of poly(lactic acid) by water-ethanol solutions. Polym. Degrad. Stab. 2018, 149, 28–38. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, W.S. Enzymatic Hydrolysis of Polylactic Acid Fiber. Appl. Biochem. Biotechnol. 2010, 164, 89–102. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, W.S. Modification of polylactic acid fabric by two lipolytic enzyme hydrolysis. Text. Res. J. 2012, 83, 229–237. [Google Scholar] [CrossRef]
- Tsuneizumi, Y.; Kuwahara, M.; Okamoto, K.; Matsumura, S. Chemical recycling of poly(lactic acid)-based polymer blends using environmentally benign catalysts. Polym. Degrad. Stab. 2010, 95, 1387–1393. [Google Scholar] [CrossRef]
- Liu, H.; Song, X.; Liu, F.; Liu, S.; Yu, S. Ferric chloride as an efficient and reusable catalyst for methanolysis of poly(lactic acid) waste. J. Polym. Res. 2015, 22, 1–7. [Google Scholar] [CrossRef]
- Okita, T.; Lee, S.H. Thermal Degradation and Biodegradability of Poly(Lactic Acid)/Corn Starch Biocomposites. J. Appl. Polym. Sci. 2006, 100, 3009–3017. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikeda, Y. Blends of Aliphatic Polyesters. III. Biodegradation of Solution-Cast Blends from Poly(L-Lactide) and Poly(ε-Caprolactone). J. Appl. Polym. Sci. 1998, 70, 2259–2268. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Tam, H.M.; Wani, S.P.; Long, T.D. Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern Vietnam. Field Crop. Res. 2006, 95, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Stloukal, P.; Kucharczyk, P. Acceleration of polylactide degradation under biotic and abiotic conditions through utilization of a new, experimental, highly compatible additive. Polym. Degrad. Stab. 2017, 142, 217–225. [Google Scholar] [CrossRef]
- Flynn, A.; Torres, L.F.; Hart-Cooper, W.; McCaffrey, Z.; Glenn, G.M.; Wood, D.F.; Orts, W.J. Evaluation of biodegradation of polylactic acid mineral composites in composting conditions. J. Appl. Polym. Sci. 2020, 137, 48939. [Google Scholar] [CrossRef]
- Sajna, V.; Nayak, S.K.; Mohanty, S. Weathering and Biodegradation Study on Graft Copolymer Compatibilized Hybrid Bionanocomposites of Poly(Lactic Acid). J. Mater. Eng. Perform. 2016, 25, 2895–2906. [Google Scholar] [CrossRef]
- Petinakis, E.; Liu, X.; Yu, L.; Way, C.; Sangwan, P.; Dean, K.; Bateman, S.; Edward, G. Biodegradation and thermal decomposition of poly(lactic acid)-based materials reinforced by hydrophilic fillers. Polym. Degrad. Stab. 2010, 95, 1704–1707. [Google Scholar] [CrossRef]
- Dharmalingam, S.; Hayes, D.G.; Wadsworth, L.C.; Dunlap, R.N.; Debruyn, J.M.; Lee, J.; Wszelaki, A.L. Soil Degradation of Polylactic Acid/Polyhydroxyalkanoate-Based Nonwoven Mulches. J. Polym. Environ. 2015, 23, 302–315. [Google Scholar] [CrossRef]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Sustainability, Compostability, and Specific Microbial Activity on Agricultural Mulch Films Prepared from Poly(lactic acid). Ind. Eng. Chem. Res. 2013, 52, 17714–17724. [Google Scholar] [CrossRef]
- Pantani, R.; Sorrentino, A. Influence of crystallinity on the biodegradation rate of injection-moulded poly(lactic acid) samples in controlled composting conditions. Polym. Degrad. Stab. 2013, 98, 1089–1096. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Tsuji, H.; Saeki, T.; Tsukegi, T.; Daimon, H.; Fujie, K. Comparative study on hydrolytic degradation and monomer recovery of poly(l-lactic acid) in the solid and in the melt. Polym. Degrad. Stab. 2008, 93, 1956–1963. [Google Scholar] [CrossRef]
- Cadar, O.; Paul, M.; Roman, C.; Miclean, M.; Majdik, C. Biodegradation Behavior of Poly(Lactic Acid) and (Lactic Acid-Ethylene Glycol-Malonic or Succinic Acid) Copolymers under Controlled Composting Conditions in a Laboratory Test System. Polym. Degrad. Stab. 2012, 97, 354–357. [Google Scholar] [CrossRef]
- The Japan Society of Mechanical Engineers, Ed. Steam Tables 1999; Maruzen Publishing: Tokyo, Japan, 1999; pp. 138–141, 158–161.
- Sato, O.; Arai, K.; Shirai, M. Hydrolysis of poly(ethylene terephthalate) and poly(ethylene 2,6-naphthalene dicarboxylate) using water at high temperature: Effect of proton on low ethylene glycol yield. Catal. Today 2006, 111, 297–301. [Google Scholar] [CrossRef]
- Iwaya, T.; Sasaki, M.; Goto, M. Kinetic analysis for hydrothermal depolymerization of nylon. Polym. Degrad. Stab. 2006, 91, 1989–1995. [Google Scholar] [CrossRef]
- Okajima, I.; Watanabe, K.; Sako, T. Depolymerization of Nylon 6 Using Subcritical Water. Kobunshi Ronbunshu 2013, 70, 731–737. [Google Scholar] [CrossRef]
- Meng, L.-H.; Zhang, Y.; Huang, Y.; Shibata, M.; Yosomiya, R. Studies on the decomposition behavior of nylon-66 in supercritical water. Polym. Degrad. Stab. 2004, 83, 389–393. [Google Scholar] [CrossRef]
- Murty, M.; Grulke, E.; Bhattacharyya, D. Influence of metallic additives on thermal degradation and liquefaction of high density polyethylene (HDPE). Polym. Degrad. Stab. 1998, 61, 421–430. [Google Scholar] [CrossRef]
- Su, L.; Wu, X.; Liu, X.; Chen, L.; Chen, K.; Hong, S. Effect of Increasing Course of Temperature and Pressure on Polypropylene Degradation in Supercritical Water. Chin. J. Chem. Eng. 2007, 15, 738–741. [Google Scholar] [CrossRef]
- Okajima, I.; Yamada, K.; Sugeta, T.; Sako, T. Waste Treatment Technologies. Decomposition of Epoxy Resin and Recycling of CFRP with Sub- and Supercritical Water. Kagaku Kogaku Ronbunshu 2002, 28, 553–558. [Google Scholar] [CrossRef]
- Tagaya, H.; Suzuki, Y.-I.; Asou, T.; Kadokawa, J.; Chiba, K. Reaction of Model Compounds of Phenol Resin and Molding Materials of Phenol Resin in Supercritical Water for Chemical Recycling of Polymer Waste. Chem. Lett. 1998, 27, 937–938. [Google Scholar] [CrossRef]
- Okajima, I.; Watanabe, K.; Haramiishi, S.; Nakamura, M.; Shimamura, Y.; Sako, T. Recycling of carbon fiber reinforced plastic containing amine-cured epoxy resin using supercritical and subcritical fluids. J. Supercrit. Fluids 2017, 119, 44–51. [Google Scholar] [CrossRef]
- Nakagawa, T.; Goto, M. Recycling thermosetting polyester resin into functional polymer using subcritical water. Polym. Degrad. Stab. 2015, 115, 16–23. [Google Scholar] [CrossRef]
- Tabasi, R.Y.; Ajji, A. Selective degradation of biodegradable blends in simulated laboratory composting. Polym. Degrad. Stab. 2015, 120, 435–442. [Google Scholar] [CrossRef]
- Haque, M.M.-U.; Puglia, D.; Fortunati, E.; Pracella, M. Effect of Reactive Functionalization on Properties and Degradability of Poly(Lactic Acid)/Poly(Vinyl Acetate) Nanocomposites with Cellulose Nanocrystals. React. Funct. Polym. 2017, 110, 1–9. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Effect of chitosan and catechin addition on the structural, thermal, mechanical and disintegration properties of plasticized electrospun PLA-PHB biocomposites. Polym. Degrad. Stab. 2016, 132, 145–156. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Abbate, C.; Arena, M.; Rizzarelli, P. Preparation, characterization and biodegradation of biopolymer nanocomposites based on fumed silica. Eur. Polym. J. 2011, 47, 139–152. [Google Scholar] [CrossRef]
- Okajima, I.; Sako, T. Recycling fiber-reinforced plastic using supercritical acetone. Polym. Degrad. Stab. 2019, 163, 1–6. [Google Scholar] [CrossRef]
- Miyata, T.; Masuko, T. Crystallization behaviour of poly(l-lactide). Polymer 1998, 39, 5515–5521. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Colloid Polym. Sci. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- ElSawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Pantani, R.; De Santis, F.; Sorrentino, A.; De Maio, F.; Titomanlio, G. Crystallization kinetics of virgin and processed poly(lactic acid). Polym. Degrad. Stab. 2010, 95, 1148–1159. [Google Scholar] [CrossRef]
- Shizuoka Prefecture Soil Fertilizer Handbook. Available online: http://www.pref.shizuoka.jp/sangyou/sa-325/hiryou/documents/sehi12-dai3bu3.pdf (accessed on 11 September 2020).









| Temperature (°C) | Pressure (MPa) | Density of Water (g/cm3) | Water (g) | PLA (g) |
|---|---|---|---|---|
| 180 | 1.003 | 0.887 | 5.85 | 0.50 |
| 200 | 1.555 | 0.865 | 5.71 | 0.50 |
| 250 | 3.978 | 0.798 | 5.27 | 0.50 |
| Reaction Temp. (°C) | Kinetic Constant kMn (min−1) |
|---|---|
| 180 | 0.2756 |
| 200 | 0.4404 |
| 250 | 1.2222 |
| Reaction Temp. (°C) | Reaction Time (min) | Heat of Crystallization (J/g) | Heat of Fusion (J/g) | Crystallization (%) |
|---|---|---|---|---|
| 180 | 5 | −15.84 | 42.74 | 19.93 |
| 180 | 10 | −13.00 | 43.32 | 22.46 |
| 180 | 15 | 0 | 45.93 | 34.02 |
| 200 | 3 | −22.19 | 44.80 | 16.75 |
| 200 | 5 | −21.38 | 46.06 | 18.28 |
| 200 | 8 | 0 | 34.34 | 25.44 |
| 250 | 2 | −11.82 | 43.97 | 23.81 |
| 250 | 3 | −1.69 | 38.03 | 26.92 |
| Before decomposition | −2.41 | 30.73 | 20.97 | |
| Reaction Temp. (°C) | Kinetic Constant kl (min−1) | Induction Time t1 (min) |
|---|---|---|
| 180 | 0.0507 | 19.8 |
| 200 | 0.0576 | 9.5 |
| 250 | 0.247 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, T.; Kishita, M.; Sun, Y.; Sako, T.; Okajima, I. Degradation of Polylactic Acid Using Sub-Critical Water for Compost. Polymers 2020, 12, 2434. https://doi.org/10.3390/polym12112434
Goto T, Kishita M, Sun Y, Sako T, Okajima I. Degradation of Polylactic Acid Using Sub-Critical Water for Compost. Polymers. 2020; 12(11):2434. https://doi.org/10.3390/polym12112434
Chicago/Turabian StyleGoto, Toshiharu, Mikitaka Kishita, Yin Sun, Takeshi Sako, and Idzumi Okajima. 2020. "Degradation of Polylactic Acid Using Sub-Critical Water for Compost" Polymers 12, no. 11: 2434. https://doi.org/10.3390/polym12112434
APA StyleGoto, T., Kishita, M., Sun, Y., Sako, T., & Okajima, I. (2020). Degradation of Polylactic Acid Using Sub-Critical Water for Compost. Polymers, 12(11), 2434. https://doi.org/10.3390/polym12112434