Morphological Changes in Astrocytes by Self-Oxidation of Dopamine to Polydopamine and Quantification of Dopamine through Multivariate Regression Analysis of Polydopamine Images
Abstract
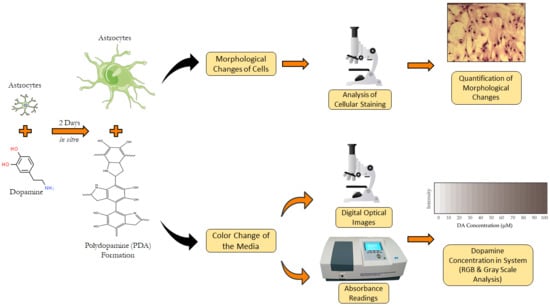
:1. Introduction
2. Materials and Methods
2.1. Synthesizing Polydopamine at pH 8.5
2.2. Preparing Astrocyte Culture Media
2.3. Treatment of Cultures of Astrocytes with Dopamine (DA)
2.4. Pretreatment of Culture Wells with DA and Polydopamine (PDA) Formation
2.5. Toxicity Response of Astrocytes to DA and PDA Formation: Cell Viability and Morphology Changes
2.6. Red, Green and Blue (RGB) and Grayscale Analysis for Detection of Dopamine Concentration in Brain Microenvironment
3. Results
3.1. Formation of PDA in the Presence of Bicarbonate Buffer at Basic pH
3.2. Morphology Changes of Astrocytes during Polymerization of DA to PDA
3.3. Effect of PDA on Astrocyte Microenvironment Prior to Plating of Cells
3.4. Estimation of DA Concentration by Novel Analysis of Images from Polymerization of DA to PDA in Astrocye Cell Culture Microenvironments
3.4.1. Mathematical Analysis of RGB Color Intensity Microscopy Images after Generation of PDA
3.4.2. Mathematical Analysis of Grayscale Intensity Images from PDA Generated from Dopamine
4. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2009, 119, 7–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Sa, Q.; Ochiai, E.; Mullins, J.; Yolken, R.; Halonen, S.K. Cerebral Toxoplasmosis: Pathogenesis, Host Resistance and Behavioural Consequences. In Toxoplasma Gondii Model Apicomplexa-Perspectives Methods, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 755–796. [Google Scholar]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Benveniste, E.N. Immune function of astrocytes. Glia 2001, 36, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Day, R.E.; Taylor, L.H.J.; Salman, M.M.; Bill, R.M.; Conner, M.T.; Conner, A.C. Identification and Molecular Mechanisms of the Rapid Tonicity-induced Relocalization of the Aquaporin 4 Channel. J. Biol. Chem. 2015, 290, 16873–16881. [Google Scholar] [CrossRef] [Green Version]
- Walz, W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int. 2000, 36, 291–300. [Google Scholar] [CrossRef]
- Guerra-Gomes, S.; Esousa, N.; Pinto, L.; Oliveira, J.F. Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front. Cell. Neurosci. 2018, 11, 427. [Google Scholar] [CrossRef] [Green Version]
- Kaczor, P.; Rakus, D.; Mozrzymas, J.W.; Kaczor, P.T. Neuron-astrocyte interaction enhance GABAergic synaptic transmission in a manner dependent on key metabolic enzymes. Front. Cell. Neurosci. 2015, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Dorszewska, J.; Prendecki, M.; Lianeri, M.; Kozubski, W. Molecular Effects of L-dopa Therapy in Parkinson’s Disease. Curr. Genom. 2014, 15, 11–17. [Google Scholar] [CrossRef]
- Brisch, R.; Esaniotis, A.; Ewolf, R.; Ebielau, H.; Ebernstein, H.-G.; Esteiner, J.; Ebogerts, B.; Braun, A.K.; Ejankowski, Z.; Ekumaratilake, J.; et al. The Role of Dopamine in Schizophrenia from a Neurobiological and Evolutionary Perspective: Old Fashioned, but Still in Vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar] [CrossRef]
- Hinshaw, S.P. Attention Deficit Hyperactivity Disorder (ADHD): Controversy, Developmental Mechanisms, and Multiple Levels of Analysis. Annu. Rev. Clin. Psychol. 2018, 14, 291–316. [Google Scholar] [CrossRef] [Green Version]
- Galloway, A.; Adeluyi, A.; O’Donovan, B.; Fisher, M.L.; Rao, C.N.; Critchfield, P.; Sajish, M.; Turner, J.R.; Ortinski, P.I. Dopamine Triggers CTCF-Dependent Morphological and Genomic Remodeling of Astrocytes. J. Neurosci. 2018, 38, 4846–4858. [Google Scholar] [CrossRef]
- Koppel, I.; Jaanson, K.; Klasche, A.; Tuvikene, J.; Tiirik, T.; Pärn, A.; Timmusk, T. Dopamine cross-reacts with adrenoreceptors in cortical astrocytes to induce BDNF expression, CREB signaling and morphological transformation. Glia 2017, 66, 206–216. [Google Scholar] [CrossRef]
- Sheng, W.; Li, B.; Wang, X.; Dai, B.; Yu, B.; Jia, X.; Zhou, F. Brushing up from “anywhere” under sunlight: A universal surface-initiated polymerization from polydopamine-coated surfaces. Chem. Sci. 2015, 6, 2068–2073. [Google Scholar] [CrossRef] [Green Version]
- Lakshminarayanan, R.; Madhavi, S.; Sim, C. Oxidative Polymerization of Dopamine: A High-Definition Multifunctional Coatings for Electrospun Nanofibers-An Overview. In Dopamine-Health and Disease; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Clement, M.-V.; Long, L.H.; Ramalingam, J.; Halliwell, B. The cytotoxicity of dopamine may be an artefact of cell culture. J. Neurochem. 2002, 81, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-P.; Liu, T.; Su, T.-L.; Liang, J. Self-Polymerization of Dopamine in Acidic Environments without Oxygen. Langmuir 2017, 33, 5863–5871. [Google Scholar] [CrossRef]
- Daniel, B.; DeCoster, M. Quantification of sPLA2-induced early and late apoptosis changes in neuronal cell cultures using combined TUNEL and DAPI staining. Brain Res. Protoc. 2004, 13, 144–150. [Google Scholar] [CrossRef]
- Wang, G.; Daniel, B.M.; DeCoster, M. Role of nitric oxide in regulating secreted phospholipase A2 release from astrocytes. NeuroReport 2005, 16, 1345–1350. [Google Scholar] [CrossRef]
- Joshi, C.; Karumuri, B.; Newman, J.J.; DeCoster, M.A. Cell morphological changes combined with biochemical assays for assessment of apoptosis and apoptosis reversal. In Current Microscopy Contributions to Advances in Science and Technology; Formatex Research Center: Badajos, Spain, 2012; pp. 756–762. [Google Scholar]
- Prats-Montalbán, J.M.; De Juan, A.; Ferrer, A. Multivariate image analysis: A review with applications. Chemom. Intell. Lab. Syst. 2011, 107, 1–23. [Google Scholar] [CrossRef]
- Geladi, P.; Grahn, H.; Esbensen, K.; Bengtsson, E. Image analysis in chemistry II. Multivariate image analysis. TrAC Trends Anal. Chem. 1992, 11, 121–130. [Google Scholar] [CrossRef]
- Vaarmann, A.; Kovac, S.H.; Holmstrom, K.M.; Gandhi, S.; Abramov, A.Y. Dopamine protects neurons against glutamate-induced excitotoxicity. Cell Death Dis. 2013, 4, e455. [Google Scholar] [CrossRef]
- Katiyar, K.S.; Winter, C.C.; Gordián-Vélez, W.J.; O’Donnell, J.C.; Song, Y.J.; Hernandez, N.S.; Struzyna, L.A.; Cullen, D.K. Three-dimensional Tissue Engineered Aligned Astrocyte Networks to Recapitulate Developmental Mechanisms and Facilitate Nervous System Regeneration. J. Vis. Exp. 2018, 2018, e55848. [Google Scholar] [CrossRef] [PubMed]
- East, E.; De Oliveira, D.B.; Golding, J.P.; Phillips, J.B. Alignment of Astrocytes Increases Neuronal Growth in Three-Dimensional Collagen Gels and Is Maintained Following Plastic Compression to Form a Spinal Cord Repair Conduit. Tissue Eng. Part. A 2010, 16, 3173–3184. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Kumazaki, M.; Fukuda, A.; Fujimoto, I.; Shimano, Y.; Hida, H.; Sakurai, T.; Deshpande, S.B.; Shimizu, H.; Morikawa, S.; et al. Acute 3-nitropropionic acid intoxication induces striatal astrocytic cell death and dysfunction of the blood-brain barrier: Involvement of dopamine toxicity. Neurosci. Res. 1997, 27, 343–355. [Google Scholar] [CrossRef]
- Yang, K.; Lee, J.S.; Kim, J.; Bin Lee, Y.; Shin, H.; Um, S.H.; Kim, J.B.; Park, K.I.; Lee, H.; Cho, S.-W. Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials 2012, 33, 6952–6964. [Google Scholar] [CrossRef]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef]
- Ku, S.H.; Park, C.B. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials 2010, 31, 9431–9437. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers. J. R. Stat. Soc. Ser. A 1995, 158, 355. [Google Scholar] [CrossRef]
- Lütkepohl, H. Handbook of Matrices, 1st ed.; Wiley: Chichester, UK, 1997. [Google Scholar]
- Heien, M.L.A.V.; Khan, A.S.; Ariansen, J.L.; Cheer, J.F.; Phillips, P.E.M.; Wassum, K.M.; Wightman, R.M. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc. Natl. Acad. Sci. USA 2005, 102, 10023–10028. [Google Scholar] [CrossRef] [Green Version]
- Owesson-White, C.A.; Roitman, M.F.; Sombers, L.A.; Belle, A.M.; Keithley, R.B.; Peele, J.L.; Carelli, R.M.; Wightman, R.M. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J. Neurochem. 2012, 121, 252–262. [Google Scholar] [CrossRef]
- Samal, S.; Dubruel, P. Cationic Polymers in Regenerative Medicine; Royal Society of Chemistry: Oxfordshire, UK, 2014. [Google Scholar]
- Darder, M.; Karan, A.; Del Real, G.; DeCoster, M.A. Cellulose-based biomaterials integrated with copper-cystine hybrid structures as catalysts for nitric oxide generation. Mater. Sci. Eng. C 2020, 108, 110369. [Google Scholar] [CrossRef]
- Canelli, E.; Mitchell, D.; Pause, R. An improved determination of chemical oxygen demand in water and wastes by a simplified acid dichromate digestion. Water Res. 1976, 10, 351–355. [Google Scholar] [CrossRef]
- LaPara, T.M.; Alleman, J.E.; Pope, P. Miniaturized closed reflux, colorimetric method for the determination of chemical oxygen demand. Waste Manag. 2000, 20, 295–298. [Google Scholar] [CrossRef]
- Haifa, B.A.; Bacârea, V.; Iacob, O.; Călinici, T.; Şchiopu, A. Comparison between digital image processing and spectrophotometric measurements methods. Application in electrophoresis interpretation. Appl. Med. Inform. 2011, 28, 29–36. [Google Scholar]
- Morosanova, M.; Bashkatova, A.; Morosanova, E. Spectrophotometric and Smartphone-Assisted Determination of Phenolic Compounds Using Crude Eggplant Extract. Molecules 2019, 24, 4407. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Astrocytes + Dopamine | ||||||
|---|---|---|---|---|---|---|
| Control | 25 µM DA | 50 µM DA | 75 µM DA | 100 µM DA | 125 µM DA | |
| Before Treatment with DA (72 h after plating) | 8.1 | 8.1 | 8.1 | 8.1 | 8.1 | 8.1 |
| After Treatment with DA (48 h post treatment) | 8.0 | 8.15 | 8.13 | 8.14 | 8.2 | 8.1 |
| ar | ag | ab | R2 | |||
|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | ||||
| Dopamine-first | 122.296 | 292.774 | −474.58 | 0.99 | 0.984 | 0.99 |
| Cells-first | 354.03 | 286.25 | −640.34 | 0.995 | 0.982 | 0.995 |
| α | β | Mean(R2) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r | g | b | r | g | b | Trial 1 | Trial 2 | Trial 3 | |
| Dopamine-first | 1 | 1 | 1 | 0.0068 | 0.009 | 0.0083 | 0.951 | 0.988 | 0.99 |
| Cells-first | 0.9899 | 0.9944 | 0.9934 | 0.0084 | 0.0101 | 0.0107 | 0.979 | 0.993 | 0.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karan, A.; Khezerlou, E.; Rezaei, F.; Iasemidis, L.; DeCoster, M.A. Morphological Changes in Astrocytes by Self-Oxidation of Dopamine to Polydopamine and Quantification of Dopamine through Multivariate Regression Analysis of Polydopamine Images. Polymers 2020, 12, 2483. https://doi.org/10.3390/polym12112483
Karan A, Khezerlou E, Rezaei F, Iasemidis L, DeCoster MA. Morphological Changes in Astrocytes by Self-Oxidation of Dopamine to Polydopamine and Quantification of Dopamine through Multivariate Regression Analysis of Polydopamine Images. Polymers. 2020; 12(11):2483. https://doi.org/10.3390/polym12112483
Chicago/Turabian StyleKaran, Anik, Elnaz Khezerlou, Farnaz Rezaei, Leon Iasemidis, and Mark A. DeCoster. 2020. "Morphological Changes in Astrocytes by Self-Oxidation of Dopamine to Polydopamine and Quantification of Dopamine through Multivariate Regression Analysis of Polydopamine Images" Polymers 12, no. 11: 2483. https://doi.org/10.3390/polym12112483
APA StyleKaran, A., Khezerlou, E., Rezaei, F., Iasemidis, L., & DeCoster, M. A. (2020). Morphological Changes in Astrocytes by Self-Oxidation of Dopamine to Polydopamine and Quantification of Dopamine through Multivariate Regression Analysis of Polydopamine Images. Polymers, 12(11), 2483. https://doi.org/10.3390/polym12112483