Development of Polysulfone Membrane via Vapor-Induced Phase Separation for Oil/Water Emulsion Filtration
Abstract
:1. Introduction
2. Materials and Methods
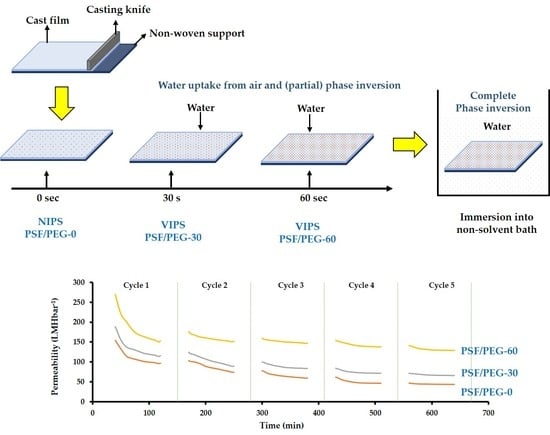
2.1. Membrane Preparation
2.2. Feed Preparation
2.3. Membrane Characterization
2.4. Filtration Setup
2.5. Membrane Fouling Resistance Test
3. Results and Discussion
3.1. Membrane Characteristics
3.1.1. Morphology
3.1.2. Mean Flow Pore Size and Pore Size Distribution
3.1.3. Surface Hydrophilicity
3.1.4. Fourier-Transform Infrared Spectra
3.1.5. Surface Chemical Composition
3.1.6. Clean Water Permeability
3.2. Oil/Water Emulsion Filtration
3.3. Membrane Fouling Resistance Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, S.; Ras, R.H.A.; Tian, X. Antifouling membranes for oily wastewater treatment: Interplay between wetting and membrane fouling. Curr. Opin. Colloid Interface Sci. 2018, 36, 90–109. [Google Scholar] [CrossRef]
- Yang, J.; Monnot, M.; Ercolei, L.; Moulin, P. Membrane-Based Processes Used in Municipal Wastewater Treatment for Water Reuse: State-of-the-Art and Performance Analysis. Membranes 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic polymer-based membrane for oily wastewater treatment: A review. Sep. Purif. Technol. 2020, 233, 1–18. [Google Scholar] [CrossRef]
- Al-Husaini, I.; Yusoff, A.; Lau, W.; Ismail, A.; Al-Abri, M.; Al-Ghafri, B.; Wirzal, M. Fabrication of polyethersulfone electrospun nanofibrous membranes incorporated with hydrous manganese dioxide for enhanced ultrafiltration of oily solution. Sep. Purif. Technol. 2019, 212, 205–214. [Google Scholar] [CrossRef]
- Putatunda, S.; Bhattacharya, S.; Sen, D.; Bhattacharjee, C. A review on the application of different treatment processes for emulsified oily wastewater. Int. J. Environ. Sci. Technol. 2018, 16, 2525–2536. [Google Scholar] [CrossRef]
- Wei, Y.; Qi, H.; Gong, X.; Zhao, S. Specially Wettable Membranes for Oil–Water Separation. Adv. Mater. Interfaces 2018, 5, 1–27. [Google Scholar] [CrossRef]
- Lin, X.; Hong, J. Recent Advances in Robust Superwettable Membranes for Oil–Water Separation. Adv. Mater. Interfaces 2019, 6, 1–23. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, G.; Bai, R.B.; Shen, S.; Zhou, X.; Wyman, I. Fabrication of superhydrophilic and underwater superoleophobic membranes via an in situ crosslinking blend strategy for highly efficient oil/water emulsion separation. J. Membr. Sci. 2019, 569, 60–70. [Google Scholar] [CrossRef]
- Esmaeili, M.; Virtanen, T.; Lahti, J.; Mänttäri, M.; Kallioinen, M. Vanillin as an Antifouling and Hydrophilicity Promoter Agent in Surface Modification of Polyethersulfone Membrane. Membranes 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.; Boels, L.; Lammertink, R.G.; De Vos, W.M. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Karim, Z.A.; Ismail, A.F. Thin Film Composite Membrane for Oily Waste Water Treatment: Recent Advances and Challenges. Membranes 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barambu, N.U.; Bilad, M.R.; Wibisono, Y.; Jaafar, J.; Mahlia, T.M.I.; Khan, A.L. Membrane Surface Patterning as a Fouling Mitigation Strategy in Liquid Filtration: A Review. Polymers 2019, 11, 1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.-H.; Xie, R.; Ju, X.-J.; Wang, W.; Liu, Z.; Chu, L.-Y. Antifouling membranes with bi-continuous porous structures and high fluxes prepared by vapor-induced phase separation. J. Membr. Sci. 2020, 611, 118256. [Google Scholar] [CrossRef]
- Ghimire, S.; Flury, M.; Scheenstra, E.J.; Miles, C.A. Sampling and degradation of biodegradable plastic and paper mulches in field after tillage incorporation. Sci. Total. Environ. 2020, 703, 135577. [Google Scholar] [CrossRef] [PubMed]
- Alammar, A.; Park, S.-H.; Williams, C.J.; Derby, B.; Szekely, G. Oil-in-water separation with graphene-based nanocomposite membranes for produced water treatment. J. Membr. Sci. 2020, 603, 118007. [Google Scholar] [CrossRef]
- Al-Shimmery, A.; Mazinani, S.; Ji, J.; Chew, Y.J.; Mattia, D. 3D printed composite membranes with enhanced anti-fouling behaviour. J. Membr. Sci. 2019, 574, 76–85. [Google Scholar] [CrossRef]
- Ikhsan, S.N.W.; Yusof, N.; Aziz, F.; Misdan, N.; Ismail, A.F.; Lau, W.-J.; Jaafar, J.; Salleh, W.N.W.; Hairom, N.H.H. Efficient separation of oily wastewater using polyethersulfone mixed matrix membrane incorporated with halloysite nanotube-hydrous ferric oxide nanoparticle. Sep. Purif. Technol. 2018, 199, 161–169. [Google Scholar] [CrossRef]
- Ding, Y.; Maruf, S.; Aghajani, M.; Greenberg, A.R. Surface patterning of polymeric membranes and its effect on antifouling characteristics. Sep. Sci. Technol. 2016, 52, 240–257. [Google Scholar] [CrossRef]
- Zoubeik, M.; Ismail, M.; Salama, A.; Henni, A. New Developments in Membrane Technologies Used in the Treatment of Produced Water: A Review. Arab. J. Sci. Eng. 2017, 43, 2093–2118. [Google Scholar] [CrossRef]
- Daramola, M.O.; Hlanyane, P.; Sadare, O.O.; Oluwasina, O.O.; Iyuke, S.E. Performance of Carbon Nanotube/Polysulfone (CNT/Psf) Composite Membranes during Oil–Water Mixture Separation: Effect of CNT Dispersion Method. Membranes 2017, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-J.; Song, H.-M.; Wang, G.; Zeng, Z.-X.; Zhao, C.-T.; Xue, Q.-J.; Guo, X.-P. Microstructures and performances of pegylated polysulfone membranes from an in situ synthesized solution via vapor induced phase separation approach. J. Colloid Interface Sci. 2018, 515, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Carretier, S.; Chen, L.-A.; Venault, A.; Yang, Z.-R.; Aimar, P.; Chang, Y. Design of PVDF/PEGMA-b-PS-b-PEGMA membranes by VIPS for improved biofouling mitigation. J. Membr. Sci. 2016, 510, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Venault, A.; Chang, Y.; Wang, D.-M.; Bouyer, D.; Higuchi, A.; Lai, J.-Y. PEGylation of anti-biofouling polysulfone membranes via liquid- and vapor-induced phase separation processing. J. Membr. Sci. 2012, 47–57. [Google Scholar] [CrossRef]
- Ismail, N.; Venault, A.; Mikkola, J.-P.; Bouyer, D.; Drioli, E.; Kiadeh, N.T.H. Investigating the potential of membranes formed by the vapor induced phase separation process. J. Membr. Sci. 2020, 597, 117601. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Chean, H.M.; Shamsuddin, N.; Bilad, M.R.; Narkkun, T.; Faungnawakij, K.; Khan, A.L. Development of Hydrophilic PVDF Membrane Using Vapour Induced Phase Separation Method for Produced Water Treatment. Membranes 2020, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Greenberg, A.; Krantz, W.B. Vapor-induced phase separation—effect of the humid air exposure step on membrane morphologyPart I. Insights from mathematical modeling. J. Membr. Sci. 2005, 258, 140–156. [Google Scholar] [CrossRef]
- Koenhen, D.M.; Mulder, M.H.V.; Smolders, C.A. Phase separation phenomena during the formation of asymmetric membranes. J. Appl. Polym. Sci. 1977, 21, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Fan, H.; Dong, Y.; Song, Y.; Han, H. Effects of exposure time on variations in the structure and hydrophobicity of polyvinylidene fluoride membranes prepared via vapor-induced phase separation. Appl. Surf. Sci. 2012, 258, 7872–7881. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F.J. Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. 2015, 132, 1–17. [Google Scholar] [CrossRef]
- Peng, Y.; Dong, Y.; Fan, H.; Chen, P.; Li, Z.; Jiang, Q. Preparation of polysulfone membranes via vapor-induced phase separation and simulation of direct-contact membrane distillation by measuring hydrophobic layer thickness. Desalination 2013, 316, 53–66. [Google Scholar] [CrossRef]
- Dehban, A.; Kargari, A.; Ashtiani, F.Z. Preparation and characterization of an antifouling poly (phenyl sulfone) ultrafiltration membrane by vapor-induced phase separation technique. Sep. Purif. Technol. 2019, 212, 986–1000. [Google Scholar] [CrossRef]
- Bilad, M.R.; Guillén, E.; Mavukkandy, M.O.; Almarzooqi, F.; Arafat, H.A. Shrinkage, defect and membrane distillation performance of composite PVDF membranes. Desalination 2015, 376, 62–72. [Google Scholar] [CrossRef]
- Pichot, R.; Spyropoulos, F.; Norton, I. O/W emulsions stabilised by both low molecular weight surfactants and colloidal particles: The effect of surfactant type and concentration. J. Colloid Interface Sci. 2010, 352, 128–135. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Zhan, X.; Han, X.-L.; Chen, C. Effect of PEG additives on properties and morphologies of polyetherimide membranes prepared by phase inversion. Front. Chem. Eng. China 2010, 4, 300–306. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes. Desalination 2011, 272, 51–58. [Google Scholar] [CrossRef]
- Lau, A.K.; Bilad, M.R.; Nordin, N.; Faungnawakij, K.; Narkkun, T.; Wang, D.K.; Mahlia, T.; Jaafar, J. Effect of membrane properties on tilted panel performance of microalgae biomass filtration for biofuel feedstock. Renew. Sustain. Energy Rev. 2020, 120, 109666. [Google Scholar] [CrossRef]
- Zhao, Z.; Ilyas, A.; Muylaert, K.; Vankelecom, I.F. Optimization of patterned polysulfone membranes for microalgae harvesting. Bioresour. Technol. 2020, 309, 123367. [Google Scholar] [CrossRef]
- Discart, V.; Bilad, M.; Moorkens, R.; Arafat, H.; Vankelecom, I.F. Decreasing membrane fouling during Chlorella vulgaris broth filtration via membrane development and coagulant assisted filtration. Algal Res. 2015, 9, 55–64. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, R.; Luo, F.; Faraj, Y.; Liu, Z.; Ju, X.-J.; Wang, W.; Chu, L.-Y. Preparation of high strength poly(vinylidene fluoride) porous membranes with cellular structure via vapor-induced phase separation. J. Membr. Sci. 2018, 549, 151–164. [Google Scholar] [CrossRef]
- Venault, A.; Chang, Y.; Wang, D.-M.; Lai, J.-Y. Surface anti-biofouling control of PEGylated poly(vinylidene fluoride) membranes via vapor-induced phase separation processing. J. Membr. Sci. 2012, 53–64. [Google Scholar] [CrossRef]
- Mulyati, S.; Aprilia, S.; Safiah; Syawaliah; A Armando, M.; Mawardi, H. The effect of poly ethylene glycol additive on the characteristics and performance of cellulose acetate ultrafiltration membrane for removal of Cr(III) from aqueous solution. IOP Conf. Series: Mater. Sci. Eng. 2018, 352, 12051. [Google Scholar] [CrossRef]
- Yadav, S.; Soontarapa, K.; Jyothi, M.S.; Padaki, M.; Geetha, B.R.; Lai, J.-Y. Supplementing multi-functional groups to polysulfone membranes using Azadirachta indica leaves powder for effective and highly selective acid recovery. J. Hazard. Mater. 2019, 369, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.S.; Nordin, N.A.H.M.; Bilad, M.R.; Aqsha, A.; Wirzal, M.D.H.; Putra, Z.A. Phase inversion and pore formation of PVDF membrane with silica as additive. J. Adv. Res. Fluid Mech. Therm. Sci. 2018, 49, 48–54. [Google Scholar]
- Bae, T.-H.; Tak, T.-M. Interpretation of fouling characteristics of ultrafiltration membranes during the filtration of membrane bioreactor mixed liquor. J. Membr. Sci. 2005, 264, 151–160. [Google Scholar] [CrossRef]
- Cho, B.; Fane, A.G. Fouling transients in nominally sub-critical flux operation of a membrane bioreactor. J. Membr. Sci. 2002, 209, 391–403. [Google Scholar] [CrossRef]
- Elhady, S.; Bassyouni, M.; Mansour, R.A.; Elzahar, M.H.; Abdel-Hamid, S.; Safaei, M.R.; Saleh, M.Y. Oily Wastewater Treatment Using Polyamide Thin Film Composite Membrane Technology. Membranes 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Arahman, N.; Mulyati, S.; Fahrina, A.; Muchtar, S.; Yusuf, M.; Takagi, R.; Matsuyama, H.; Nordin, N.A.H.; Bilad, M.R. Improving Water Permeability of Hydrophilic PVDF Membrane Prepared via Blending with Organic and Inorganic Additives for Humic Acid Separation. Molecules 2019, 24, 4099. [Google Scholar] [CrossRef] [Green Version]
- Mazinani, S.; Al-Shimmery, A.; Chew, Y.M.J.; Mattia, D. 3D Printed Fouling-Resistant Composite Membranes. ACS Appl. Mater. Interfaces 2019, 11, 26373–26383. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Hu, Y.; Sun, S. Membrane technology in wastewater treatment enhanced by functional nanomaterials. J. Clean. Prod. 2018, 197, 339–348. [Google Scholar] [CrossRef]











| Membrane | Composition (wt%) | ||
|---|---|---|---|
| C | O | S | |
| PSF/PEG-0 | 70.58 | 24.64 | 4.78 |
| PSF/PEG-30 | 69.40 | 25.86 | 4.74 |
| PSF/PEG-60 | 69.02 | 26.26 | 4.72 |
| Membrane | Surface Elemental (mol%) | |||
|---|---|---|---|---|
| O 1s | C 1s | S 2p | O 1s/C 1s | |
| PSF/PEG-0 | 16.99 | 81.49 | 1.52 | 0.208 |
| PSF/PEG-60 | 17.96 | 80.16 | 1.88 | 0.229 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barambu, N.U.; Bilad, M.R.; Bustam, M.A.; Huda, N.; Jaafar, J.; Narkkun, T.; Faungnawakij, K. Development of Polysulfone Membrane via Vapor-Induced Phase Separation for Oil/Water Emulsion Filtration. Polymers 2020, 12, 2519. https://doi.org/10.3390/polym12112519
Barambu NU, Bilad MR, Bustam MA, Huda N, Jaafar J, Narkkun T, Faungnawakij K. Development of Polysulfone Membrane via Vapor-Induced Phase Separation for Oil/Water Emulsion Filtration. Polymers. 2020; 12(11):2519. https://doi.org/10.3390/polym12112519
Chicago/Turabian StyleBarambu, Nafiu Umar, Muhammad Roil Bilad, Mohamad Azmi Bustam, Nurul Huda, Juhana Jaafar, Thanitporn Narkkun, and Kajornsak Faungnawakij. 2020. "Development of Polysulfone Membrane via Vapor-Induced Phase Separation for Oil/Water Emulsion Filtration" Polymers 12, no. 11: 2519. https://doi.org/10.3390/polym12112519
APA StyleBarambu, N. U., Bilad, M. R., Bustam, M. A., Huda, N., Jaafar, J., Narkkun, T., & Faungnawakij, K. (2020). Development of Polysulfone Membrane via Vapor-Induced Phase Separation for Oil/Water Emulsion Filtration. Polymers, 12(11), 2519. https://doi.org/10.3390/polym12112519