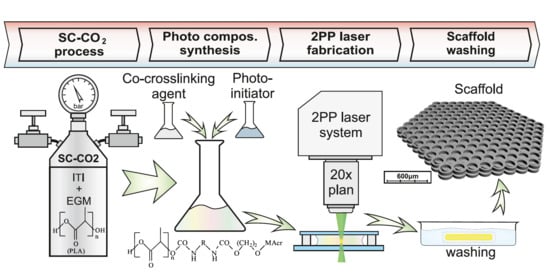
Photocurable Methacrylate Derivatives of Polylactide: A Two-Stage Synthesis in Supercritical Carbon Dioxide and 3D Laser Structuring
Abstract
:1. Introduction
2. Materials and Methods
2.1. PLA Modification Process
2.2. Preparation of Crosslinked Structures
2.3. Studies of Mechanical Characteristics
2.4. Differential Thermal Analysis
2.5. Cell Culture
2.6. Cytotoxicity Assessment
3. Results and Discussion
3.1. A Two-Stage Synthesis of Polylactide Modified with Functional Groups in the scCO2 Medium
3.2. The Effect of the Crosslinking Agent (OUDM) Concentration on the Structure of Photocured Compositions
3.3. Study of the Local Stiffness of Crosslinked Structures
3.4. DTA Study of Crosslinked Samples
3.5. Cytotoxicity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knight, R.L.; Wilcox, H.E.; Korossis, S.A.; Fisher, J.; Ingham, E. The use of acellular matrices for the tissue engineering of cardiac valves. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 129–143. [Google Scholar] [CrossRef]
- Shakhssalim, N.; Soleimani, M.; Dehghan, M.M.; Rasouli, J.; Taghizadeh-Jahed, M.; Torbati, P.M.; Naji, M. Bladder smooth muscle cells on electrospun poly(ε-caprolactone)/poly(l-lactic acid) scaffold promote bladder regeneration in a canine model. Mater. Sci. Eng. C 2017, 75, 877–884. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, T.; Fu, W.; Zhang, H. Electrospun polycaprolactone/collagen nanofibers cross-linked with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide and genipin facilitate endothelial cell regeneration and may be a promising candidate for vascular scaffolds. Int. J. Nanomed. 2019, 14, 2127–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, T.S.; Chakrapani, V.; Natesan, P.V.; Raj, D.K.; Kumary, T.V. Effect of Molecular Weight on Electro Spun Pcl Based Composite Fibrous Mats. Biomed. J. Sci. Tech. Res. 2017, 1, 1127. [Google Scholar] [CrossRef] [Green Version]
- Giammona, G.; Craparo, E.F. Biomedical Applications of Polylactide (PLA) and Its Copolymers. Molecules 2018, 23, 980. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-F.; Hong, J.; Zheng, Q.; Guo, X.; Lan, S.; Cui, F.; Pan, H.; Zou, Z.; Chen, C. Repair of rat cranial bone defects with nHAC/PLLA and BMP-2-related peptide or rhBMP-2. J. Orthop. Res. 2011, 29, 1745–1752. [Google Scholar] [CrossRef]
- Wang, S.; Wan, Y.; Cai, Q.; He, B.; Chen, W. Molecular design of synthetic biodegradable polymers as cell scaffold materials. Chem. Res. Chin. U 2004, 20, 191–194. [Google Scholar] [CrossRef]
- Gruber, P.; Drumright, R.E.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841. [Google Scholar]
- Rytlewski, P.; Mróz, W.; Żenkiewicz, M.; Czwartos, J.; Budner, B. Laser induced surface modification of polylactide. J. Mater. Process. Technol. 2012, 212, 1700–1704. [Google Scholar] [CrossRef]
- Rasal, R.M.; Hirt, D.E. Toughness decrease of PLA-PHBHHx blend films upon surface-confined photopolymerization. J. Biomed. Mater. Res. Part A 2009, 88, 1079–1086. [Google Scholar] [CrossRef]
- Thakur, V.K.; Vennerberg, D.; Kessler, M.R. Green Aqueous Surface Modification of Polypropylene for Novel Polymer Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 9349–9356. [Google Scholar] [CrossRef] [Green Version]
- Mahjoubi, H.; Kinsella, J.M.; Murshed, M.; Cerruti, M. Surface Modification of Poly(d,l-Lactic Acid) Scaffolds for Orthopedic Applications: A Biocompatible, Nondestructive Route via Diazonium Chemistry. ACS Appl. Mater. Interfaces 2014, 6, 9975–9987. [Google Scholar] [CrossRef]
- Wu, H.; Gou, Y.; Wang, J.; Tao, L. Multicomponent Reactions for Surface Modification. Macromol. Rapid Commun. 2018, 39, e1800064. [Google Scholar] [CrossRef] [PubMed]
- El Habnouni, S.; Lavigne, J.-P.; Darcos, V.; Porsio, B.; Garric, X.; Coudane, J.; Nottelet, B. Toward potent antibiofilm degradable medical devices: A generic method for the antibacterial surface modification of polylactide. Acta Biomater. 2013, 9, 7709–7718. [Google Scholar] [CrossRef] [PubMed]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, X.; Deng, M.; Hong, Z.; Luo, R.; Chen, X.; Jing, X. The starch grafted poly(l-lactide) and the physical properties of its blending composites. Polymer 2005, 46, 5723–5729. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Morphology and properties of compatibilized polylactide/thermoplastic starch blends. Polymer 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Popelka, Š.; Machova, L.; Rypacek, F. Adsorption of poly(ethylene oxide)–block–polylactide copolymers on polylactide as studied by ATR-FTIR spectroscopy. J. Colloid Interface Sci. 2007, 308, 291–299. [Google Scholar] [CrossRef]
- Akopova, T.A.; Timashev, P.S.; Demina, T.; Bardakova, K.N.; Minaev, N.V.; Burdukovskii, V.F.; Cherkaev, G.V.; Vladimirov, L.V.; Istomin, A.V.; Svidchenko, E.A.; et al. Solid-state synthesis of unsaturated chitosan derivatives to design 3D structures through two-photon-induced polymerization. Mendeleev Commun. 2015, 25, 280–282. [Google Scholar] [CrossRef]
- Demina, T.; Bardakova, K.N.; Minaev, N.V.; Svidchenko, E.A.; Istomin, A.V.; Goncharuk, G.P.; Vladimirov, L.V.; Grachev, A.V.; Zelenetskii, A.N.; Timashev, P.; et al. Two-Photon-Induced Microstereolithography of Chitosan-g-Oligolactides as a Function of Their Stereochemical Composition. Polymer 2017, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Melissinaki, V.; Gill, A.A.; Ortega, I.; Vamvakaki, M.; Ranella, A.; Haycock, J.W.; Fotakis, C.; Farsari, M.; Claeyssens, F. Direct laser writing of 3D scaffolds for neural tissue engineering applications. Biofabrication 2011, 3, 045005. [Google Scholar] [CrossRef]
- Helminen, A.O.; Korhonen, H.; Seppälä, J.V. Structure modification and crosslinking of methacrylated polylactide oligomers. J. Appl. Polym. Sci. 2002, 86, 3616–3624. [Google Scholar] [CrossRef]
- Sakai, R.; John, B.; Okamoto, M.; Seppälä, J.; Vaithilingam, J.; Hussein, H.; Goodridge, R. Fabrication of Polylactide-Based Biodegradable Thermoset Scaffolds for Tissue Engineering Applications. Macromol. Mater. Eng. 2012, 298, 45–52. [Google Scholar] [CrossRef]
- Helminen, A.O.; Korhonen, H.; Seppälä, J.V. Cross-Linked Poly(-caprolactone/D,L-lactide) Copolymers with Elastic Properties. Macromol. Chem. Phys. 2002, 203, 2630–2639. [Google Scholar] [CrossRef]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Giammona, G. Photocrosslinkable polyaspartamide/polylactide copolymer and its porous scaffolds for chondrocytes. Mater. Sci. Eng. C 2017, 76, 794–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Declercq, H.; Cornelissen, M.; Gorskiy, T.L.; Schacht, E.H. Osteoblast behaviour on in situ photopolymerizable three-dimensional scaffolds based on D, L-lactide, ε-caprolactone and trimethylene carbonate. J. Mater. Sci. Mater. Electron. 2006, 17, 113–122. [Google Scholar] [CrossRef]
- Ronca, A.; Ambrosio, L.; Grijpma, D.W. Design of porous three-dimensional PDLLA/nano-hap composite scaffolds using stereolithography. J. Appl. Biomater. Funct. Mater. 2012, 10, 249–258. [Google Scholar] [CrossRef]
- Shashkova, V.T.; Matveeva, I.A.; Glagolev, N.N.; Zarkhina, T.S.; Cherkasova, A.V.; Kotova, S.; Timashev, P.S.; Solovieva, A.B. Synthesis of polylactide acrylate derivatives for the preparation of 3D structures by photo-curing. Mendeleev Commun. 2016, 26, 418–420. [Google Scholar] [CrossRef]
- Amsden, B.G.; Misra, G.; Gu, F.; Younes, H.M. Synthesis and Characterization of A Photo-Cross-Linked Biodegradable Elastomer. Biomacromolecules 2004, 5, 2479–2486. [Google Scholar] [CrossRef]
- Żółtowska, K.; Piotrowska, U.; Oledzka, E.; Kuras, M.J.; Zgadzaj, A.; Sobczak, M. Biodegradable Poly(ester-urethane) Carriers Exhibiting Controlled Release of Epirubicin. Pharm. Res. 2017, 34, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Mândru, M.; Ciobanu, C.; Vlad, S.; Butnaru, M.; Lebrun, L.; Popa, M. Characteristics of polyurethane-based sustained release membranes for drug delivery. Open Chem. 2013, 11, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Wang, G.; Neumann, K.; Yao, W.; Ding, L.; Li, S.; Sheng, Y.; Jiang, Y.; Bradley, M.; Zhang, R. Synthesis and characterization of biodegradable poly(ether-ester) urethane acrylates for controlled drug release. Mater. Sci. Eng. C 2017, 74, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shashkova, V.T.; Matveeva, I.A.; Glagolev, N.N.; Zarkhina, T.S.; Timashev, P.S.; Bagratashvili, V.N.; Solov’Eva, A.B. Selective modification of polylactide by introducing acrylate groups: IR spectroscopy, gel permeation chromatography, and differential thermal analysis. Russ. J. Phys. Chem. A 2016, 90, 1925–1930. [Google Scholar] [CrossRef]
- Schnabelrauch, M.; Vogt, S.; Larcher, Y.; Wilke, I. Biodegradable polymer networks based on oligolactide macromers: Synthesis, properties and biomedical applications. Biomol. Eng. 2002, 19, 295–298. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Mase, N.; Uzzaman, M.; Yamamoto, S.; Nakaya, Y.; Sato, K.; Narumi, T. Organocatalytic Stereoselective Cyclic Polylactide Synthesis in Supercritical Carbon Dioxide under Plasticizing Conditions. Polymers 2018, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Naylor, A.; Timashev, P.S.; Solov’Eva, A.B.; Erina, N.A.; Kotova, S.; Busby, A.J.; Popov, V.K.; Howdle, S. Can Supercritical Carbon Dioxide Improve the Mechanical Integrity of Ultrahigh-Molecular-Weight Polyethylene? Adv. Mater. 2008, 20, 575–578. [Google Scholar] [CrossRef]
- Naylor, A.; Howdle, S.M. The preparation of novel blends of Ultra High Molecular Weight Polyethylene with polymethacrylate based copolymers using supercritical carbon dioxide. J. Mater. Chem. 2005, 15, 5037–5042. [Google Scholar] [CrossRef]
- Timashev, P.S.; Vorobieva, N.N.; Akovantseva, A.A.; Minaev, N.V.; Piskun, Y.A.; Kostjuk, S.V.; Selezneva, I.I.; Vasilenko, I.V.; Zakharkina, O.L.; Ignatieva, N.Y.; et al. Biocompatibility and Degradation of Porous Matrixes from Lactide and ε-Caprolactone Copolymers Formed in a Supercritical Carbon Dioxide Medium. Russ. J. Phys. Chem. B 2017, 11, 1095–1102. [Google Scholar] [CrossRef]
- Timashev, P.S.; Vorobieva, N.N.; Minaev, N.V.; Piskun, Y.A.; Vasilenko, I.V.; Lakeev, S.G.; Kostyuk, S.V.; Lunin, V.V.; Bagratashvili, V.N. Formation of porous matrices from lactide and ε-caprolactone copolymers in supercritical carbon dioxide medium. Russ. J. Phys. Chem. B 2016, 10, 1195–1200. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, A.L.; García-González, C. Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO₂-Based Approach. Molecules 2019, 24, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudova, D.S.; Bardakova, K.N.; Akopova, T.A.; Timashev, P.S.; Minaev, N.V. Features of structures formation on the basis of chitosan derivatives by a prototype of 263 nm laser stereolithograph. J. Phys. Conf. Ser. 2016, 737, 12046. [Google Scholar] [CrossRef]
- Demina, T.; Bardakova, K.N.; Svidchenko, E.A.; Minaev, N.V.; Pudovkina, G.I.; Novikov, M.M.; Butnaru, D.; Surin, N.M.; Akopova, T.A.; Bagratashvili, V.N.; et al. Fabrication of microstructured materials based on chitosan and D,L-lactide copolymers using laser-induced microstereolithography. High Energy Chem. 2016, 50, 389–394. [Google Scholar] [CrossRef]
- Timashev, P.S.; Bardakova, K.N.; Minaev, N.V.; Demina, T.; Mishchenko, T.A.; Mitroshina, E.V.; Akovantseva, A.A.; Koroleva, A.V.; Asyutin, D.S.; Pimenova, L.F.; et al. Compatibility of cells of the nervous system with structured biodegradable chitosan-based hydrogel matrices. Appl. Biochem. Microbiol. 2016, 52, 508–514. [Google Scholar] [CrossRef]
- Zurina, I.M.; Shpichka, A.; Saburina, I.N.; Kosheleva, N.V.; Gorkun, A.A.; Grebenik, E.A.; Kuznetsova, D.S.; Zhang, D.; Rochev, Y.A.; Butnaru, D.; et al. 2D/3D buccal epithelial cell self-assembling as a tool for cell phenotype maintenance and fabrication of multilayered epithelial linings in vitro. Biomed. Mater. 2018, 13, 054104. [Google Scholar] [CrossRef] [PubMed]
- Shpichka, A.I.; Koroleva, A.; Kuznetsova, D.; Dmitriev, R.I.; Timashev, P. Fabrication and handling of 3D scaffolds based on polymers and decellularized tissues, Multi-Parametric Live Cell Microscopy of 3D Tissue Models. Adv. Exp. Med. Biol. 2017, 1035, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.; Ageykin, A.; Koroleva, A.; Deiwick, A.; Shpichka, A.I.; Solovieva, A.; Kostjuk, S.; Meleshina, A.; Rodimova, S.; Akovanceva, A.; et al. Surface micromorphology of cross-linked tetrafunctional polylactide scaffolds inducing vessel growth and bone formation. Biofabrication 2017, 9, 025009. [Google Scholar] [CrossRef]
- Koroleva, A.; Deiwick, A.; Nguyen, A.; Schlie-Wolter, S.; Narayan, R.; Timashev, P.; Popov, V.; Bagratashvili, V.N.; Chichkov, B.N. Osteogenic Differentiation of Human Mesenchymal Stem Cells in 3-D Zr-Si Organic-Inorganic Scaffolds Produced by Two-Photon Polymerization Technique. PLoS ONE 2015, 10, e0118164. [Google Scholar] [CrossRef]
- Timashev, P.; Kuznetsova, D.; Koroleva, A.; Prodanets, N.; Deiwick, A.; Piskun, Y.; Bardakova, K.; Dzhoyashvili, N.; Kostjuk, S.; Zagaynova, E.; et al. Novel biodegradable star-shaped polylactide scaffolds for bone regeneration fabricated by two-photon polymerization. Nanomedicine 2016, 11, 1041–1053. [Google Scholar] [CrossRef]
- Uglea, C.V. Oligomer Technology and Applications; CRC Press: Clemson, SC, USA, 1998. [Google Scholar] [CrossRef]
- Saniei, H.; Mousavi, S. Surface modification of PLA 3D-printed implants by electrospinning with enhanced bioactivity and cell affinity. Polymer 2020, 196, 122467. [Google Scholar] [CrossRef]
- Evlashin, S.; Dyakonov, P.; Tarkhov, M.; Dagesyan, S.; Rodionov, S.; Shpichka, A.; Kostenko, M.; Konev, S.; Sergeichev, I.; Timashev, P.; et al. Flexible Polycaprolactone and Polycaprolactone/Graphene Scaffolds for Tissue Engineering. Materials 2019, 12, 2991. [Google Scholar] [CrossRef] [Green Version]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’Heureux, N.; Fricain, J.-C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2017, 106, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Rotshteyn, Y.; Rabadi, L.; Parikh, R.; Bullock, P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicol. Vitr. 2004, 18, 703–710. [Google Scholar] [CrossRef] [PubMed]















| No. | Technique of Photocuring * | Δm 400–500 °C, % | WMax, %/min | % of Coke Residue at 500 °C |
|---|---|---|---|---|
| 1 | Laser stereolithography (λ = 263 nm, 0.2 W/cm2) | 6 | 0.7 | 15 |
| 2 | LED photocuring of a film (λ = 365 nm, 5 W/cm2) | 11 | 2.7 | 9 |
| 3 | DRT-1000 lamp photocuring of a film (λ = 253 nm, ≈0.03 W/cm2) | 15 | 4.4 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplin, V.S.; Glagolev, N.N.; Shashkova, V.T.; Matveeva, I.A.; Shershnev, I.V.; Zarkhina, T.S.; Minaev, N.V.; Aksenova, N.A.; Shavkuta, B.S.; Bezrukov, E.A.; et al. Photocurable Methacrylate Derivatives of Polylactide: A Two-Stage Synthesis in Supercritical Carbon Dioxide and 3D Laser Structuring. Polymers 2020, 12, 2525. https://doi.org/10.3390/polym12112525
Kaplin VS, Glagolev NN, Shashkova VT, Matveeva IA, Shershnev IV, Zarkhina TS, Minaev NV, Aksenova NA, Shavkuta BS, Bezrukov EA, et al. Photocurable Methacrylate Derivatives of Polylactide: A Two-Stage Synthesis in Supercritical Carbon Dioxide and 3D Laser Structuring. Polymers. 2020; 12(11):2525. https://doi.org/10.3390/polym12112525
Chicago/Turabian StyleKaplin, Vladislav S., Nikolay N. Glagolev, Valentina T. Shashkova, Irina A. Matveeva, Ilya V. Shershnev, Tatyana S. Zarkhina, Nikita V. Minaev, Nadezhda A. Aksenova, Boris S. Shavkuta, Evgeny A. Bezrukov, and et al. 2020. "Photocurable Methacrylate Derivatives of Polylactide: A Two-Stage Synthesis in Supercritical Carbon Dioxide and 3D Laser Structuring" Polymers 12, no. 11: 2525. https://doi.org/10.3390/polym12112525
APA StyleKaplin, V. S., Glagolev, N. N., Shashkova, V. T., Matveeva, I. A., Shershnev, I. V., Zarkhina, T. S., Minaev, N. V., Aksenova, N. A., Shavkuta, B. S., Bezrukov, E. A., Kopylov, A. S., Kuznetsova, D. S., Shpichka, A. I., Timashev, P. S., & Solovieva, A. B. (2020). Photocurable Methacrylate Derivatives of Polylactide: A Two-Stage Synthesis in Supercritical Carbon Dioxide and 3D Laser Structuring. Polymers, 12(11), 2525. https://doi.org/10.3390/polym12112525