Poly(N-Isopropylacrylamide)-Functional Silicon Nanocrystals for Thermosensitive Fluorescence Cellar Imaging
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of PNIPAAm-Functional Si NCs
2.2.1. Synthesis of Si NCs
2.2.2. Synthesis of Folic Acid Modified Silicon NCs (Si NCs-FA)
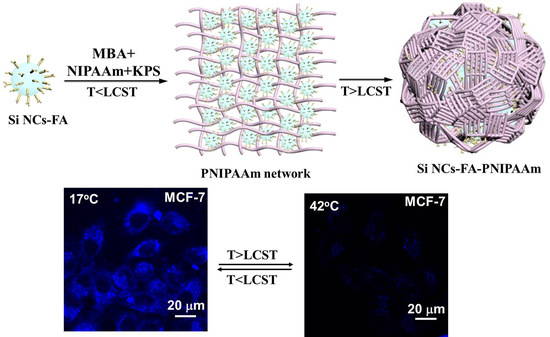
2.2.3. Synthesis of Temperature Sensitive N-Isopropylacrylamide (PNIPAAm)-Coated Si NCs-FA (Si NCs-FA-PNIPAAm)
2.3. Characterization
2.4. Cell Culture
2.5. Cell Biocompatibility Assay
2.6. Cell Imaging
3. Results and Discussion
3.1. Characterization of Fluorescent Probes
3.2. Optical Properties of the Fluorescent Probes
3.3. Application of the Fluorescent Probes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Mandal, R.; Ratchford, D.C.; Anthony, R.; Yeom, J. Si Nanocrystals/ZnO nanowires hybrid structures as immobilized photocatalysts for photodegradation. Nanomaterials 2020, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Fujii, M.; Fujii, R.; Takada, M.; Sugimoto, H. Silicon quantum dot supraparticles for fluorescence bioimaging. ACS Appl. Nano Mater. 2020, 3, 6099–6107. [Google Scholar] [CrossRef]
- Nguyen, A.; Gonzalez, C.M.; Sinelnikov, R.; Newman, W.; Sun, S.; Lockwood, R.; Veinot, J.G.; Meldrum, A. Detection of nitroaromatics in the solid, solution, and vapor phases using silicon quantum dot sensors. Nanotechnology 2016, 27, 105501. [Google Scholar] [CrossRef]
- Romano, F.; Angeloni, S.; Morselli, G.; Mazzaro, R.; Morandi, V.; Shell, J.R.; Cao, X.; Pogue, B.W.; Ceroni, P. Water-soluble silicon nanocrystals as NIR luminescent probes for time-gated biomedical imaging. Nanoscale 2020, 12, 7921–7926. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Multifunctional mesoporous silica nanoplatform based on silicon nanoparticles for targeted two-photon-excited fluorescence imaging-guided chemo/photodynamic synergetic therapy in vitro. Talanta 2020, 209, 120552. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.; Pi, X.; Yang, D. Light-emitting diodes based on colloidal silicon quantum dots. J. Semicond. 2018, 39, 061008. [Google Scholar] [CrossRef]
- Cho, E.C.; Park, S.; Hao, X.; Song, D.; Conibeer, G.; Park, S.C.; Green, M.A. Silicon quantum dot/crystalline silicon solar cells. Nanotechnology 2008, 19, 245201. [Google Scholar] [CrossRef]
- Liu, C.Y.; Holman, Z.C.; Kortshagen, U.R. Hybrid solar cells from P3HT and silicon nanocrystals. Nano Lett. 2009, 9, 449–452. [Google Scholar] [CrossRef]
- Qiao, J.; Hwang, Y.H.; Kim, D.P.; Qi, L. Simultaneous monitoring of temperature and Ca(2+) concentration variation by fluorescent polymer during intracellular heat production. Anal. Chem. 2020, 92, 8579–8583. [Google Scholar] [CrossRef]
- Liu, Z.; Jing, X.; Zhang, S.; Tian, Y. A copper nanocluster-based fluorescent probe for real-time imaging and ratiometric biosensing of calcium ions in neurons. Anal. Chem. 2019, 91, 2488–2497. [Google Scholar] [CrossRef]
- Dou, Y.K.; Shang, Y.; He, X.W.; Li, W.Y.; Li, Y.H.; Zhang, Y.K. Preparation of a ruthenium-complex-functionalized two-photon-excited red fluorescence silicon nanoparticle composite for targeted fluorescence imaging and photodynamic therapy in vitro. ACS Appl. Mater. Interfaces 2019, 11, 13954–13963. [Google Scholar] [CrossRef]
- Wu, J.; Li, B.; Feng, Y.; Shao, Y.; Wu, X.; Sun, Y. Silicon quantum dot- assisted synthesis of MoS2/rGO sandwich structures with excellent supercapacitive performance. New J. Chem. 2019, 43, 8660–8668. [Google Scholar] [CrossRef]
- Hill, S.K.E.; Connell, R.; Peterson, C.; Hollinger, J.; Hillmyer, M.A.; Kortshagen, U.; Ferry, V.E. Silicon quantum dot-poly(methyl methacrylate) nanocomposites with reduced light scattering for luminescent solar concentrators. ACS Photonics 2019, 6, 170–180. [Google Scholar] [CrossRef]
- Islam, M.A.; Purkait, T.K.; Mobarok, M.H.; Hoehlein, I.M.D.; Sinelnikov, R.; Iqbal, M.; Azulay, D.; Balberg, I.; Millo, O.; Rieger, B.; et al. Grafting poly(3-hexylthiophene) from silicon nanocrystal surfaces: Synthesis and properties of a functional hybrid material with direct interfacial contact. Angew. Chem. Int. Ed. 2016, 55, 7393–7397. [Google Scholar] [CrossRef] [PubMed]
- Dasog, M.; Kehrle, J.; Rieger, B.; Veinot, J.G.C. Silicon nanocrystals and silicon-polymer hybrids: Synthesis, surface engineering, and applications. Angew. Chem. Int. Ed. 2016, 55, 2322–2339. [Google Scholar] [CrossRef]
- Sacarescu, L.; Roman, G.; Sacarescu, G.; Simionescu, M. Fluorescence detection system based on silicon quantum dots–polysilane nanocomposites. Express Polym. Lett. 2016, 10, 990–1002. [Google Scholar] [CrossRef]
- Hessel, C.M.; Rasch, M.R.; Hueso, J.L.; Goodfellow, B.W.; Akhavan, V.A.; Puvanakrishnan, P.; Tunnel, J.W.; Korgel, B.A. Alkyl Passivation and amphiphilic polymer coating of silicon nanocrystals for diagnostic imaging. Small 2010, 6, 2026–2034. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.K.E.; Connell, R.; Held, J.; Peterson, C.; Francis, L.; Hillmyer, M.A.; Ferry, V.E.; Kortshagen, U. Poly(methyl methacrylate) films with high concentrations of silicon quantum dots for visibly transparent luminescent solar concentrators. ACS Appl. Mater. Interfaces 2020, 12, 4572–4578. [Google Scholar] [CrossRef]
- Cheng, C.; Wei, H.; Shi, B.X.; Cheng, H.; Li, C.; Gu, Z.W.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Biotinylated thermoresponsive micelle self-assembled from double-hydrophilic block copolymer for drug delivery and tumor target. Biomaterials 2008, 29, 497–505. [Google Scholar] [CrossRef]
- Wang, X.; Xu, K.; Yao, H.; Chang, L.; Wang, Y.; Li, W.; Zhao, Y.; Qin, J. Temperature-regulated aggregation-induced emissive self-healable hydrogels for controlled drug delivery. Polym. Chem. 2018, 9, 5002–5013. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, W.; Zhang, Z.; Chen, M.; Wang, J.; Qian, X.; Qin, W. Sensitive western-blot analysis of azide-tagged protein post translational modifications using thermoresponsive polymer self-assembly. Anal. Chem. 2018, 90, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.L.; Cai, S.J.; Li, S.; He, X.W.; Li, W.Y.; Li, Y.H.; Zhang, Y.K. One-pot microwave synthesis of water-dispersible, high fluorescence silicon nanoparticles and their imaging applications in vitro and in vivo. Anal. Chem. 2016, 88, 11631–11638. [Google Scholar] [CrossRef]
- Joo, K.I.; Fang, Y.; Liu, Y.; Xiao, L.; Gu, Z.; Tai, A.; Lee, C.L.; Tang, Y.; Wang, P. Enhanced real-time monitoring of adeno-associated virus trafficking by virus–quantum dot conjugates. ACS Nano 2011, 5, 3523–3535. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Qian, X.; Zhang, L.; Miao, W.; Ming, D.; Jiang, L.; Huang, H. Enhanced imaging of glycan expressing cancer cells using poly(glycidyl methacrylate)-grafted silica nanospheres labeled with quantum dots. Anal. Chim. Acta 2020, 1095, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hong, X.; Liu, Y.; Li, D.; Wang, Y.W.; Li, J.H.; Bai, Y.B.; Li, T.J. Highly photoluminescent CdTe/poly(N-isopropylacrylamide) temperature-sensitive gels. Adv. Mater. 2005, 17, 163–166. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, Y.Y.; Zhang, F.; Li, W.Y. One-pot synthesis of thermal responsive QDs–PNIPAM hybrid fluorescent microspheres by controlling the polymerization temperature at two different polymerization stages. J. Mater. Chem. 2011, 21, 6556. [Google Scholar] [CrossRef]
- Jańczewski, D.; Tomczak, N.; Han, M.Y.; Vancso, G.J. Introduction of quantum dots into PNIPAM microspheres by precipitation polymerization above LCST. Eur. Polym. J. 2009, 45, 1912–1917. [Google Scholar] [CrossRef]
- Lin, S.W.; Chen, D.H. Synthesis of water-soluble blue photoluminescent silicon nanocrystals with oxide surface passivation. Small 2009, 5, 72–76. [Google Scholar] [CrossRef]
- Neves, D.A.; Lobato, K.B.; Angelica, R.S.; Teixeira Filho, J.; Oliveira, G.P.R.; Godoy, H.T. Thermal and in vitro digestion stability of folic acid in bread. J. Food Compos. Anal. 2019, 84, 103311. [Google Scholar] [CrossRef]
- Ding, X.; Yao, P. Soy protein/soy polysaccharide complex nanogels: Folic acid loading, protection, and controlled delivery. Langmuir 2013, 29, 8636–8644. [Google Scholar] [CrossRef]
- Linden, H.J.; Herbev, S.; Olthuis, W.; Bergveld, P. Stimulus-sensitive hydrogels and their applications in chemical (micro)analysis. Analyst 2003, 128, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Yan, X.P. Doped quantum dots for chemo/biosensing and bioimaging. Chem. Soc. Rev. 2013, 42, 5489–5521. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, D.; Fan, Z.; Huang, Z.; Mao, H.; Ma, Y. One-step hydrothermal synthesis of ultrabright water-soluble silicon nanoparticles for folate-receptor-mediated bioimaging. J. Mater. Sci. 2019, 54, 9707–9717. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, L.; Shi, Y.; Huang, J.; Yang, Y.; Ming, D. Poly(N-Isopropylacrylamide)-Functional Silicon Nanocrystals for Thermosensitive Fluorescence Cellar Imaging. Polymers 2020, 12, 2565. https://doi.org/10.3390/polym12112565
Li Y, Zhang L, Shi Y, Huang J, Yang Y, Ming D. Poly(N-Isopropylacrylamide)-Functional Silicon Nanocrystals for Thermosensitive Fluorescence Cellar Imaging. Polymers. 2020; 12(11):2565. https://doi.org/10.3390/polym12112565
Chicago/Turabian StyleLi, Yiting, Lihui Zhang, Youhong Shi, Jialing Huang, Yaqiong Yang, and Dengming Ming. 2020. "Poly(N-Isopropylacrylamide)-Functional Silicon Nanocrystals for Thermosensitive Fluorescence Cellar Imaging" Polymers 12, no. 11: 2565. https://doi.org/10.3390/polym12112565
APA StyleLi, Y., Zhang, L., Shi, Y., Huang, J., Yang, Y., & Ming, D. (2020). Poly(N-Isopropylacrylamide)-Functional Silicon Nanocrystals for Thermosensitive Fluorescence Cellar Imaging. Polymers, 12(11), 2565. https://doi.org/10.3390/polym12112565