Evaluation of Glycerylphytate Crosslinked Semi- and Interpenetrated Polymer Membranes of Hyaluronic Acid and Chitosan for Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Methacrylated Hyaluronic Acid
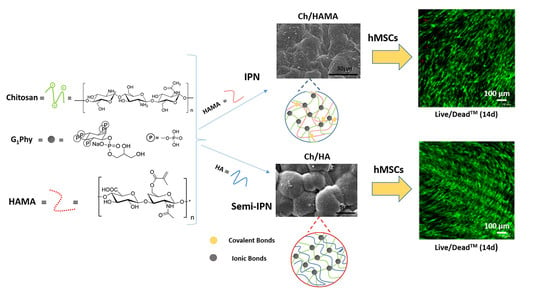
2.3. Preparation of Ch Membranes
2.4. Preparation of Ch/HA and Ch/HAMA Membranes
2.5. Characterisation Techniques
2.6. G1Phy Quantification
2.7. Swelling
2.8. Degradation
2.9. Release of G1Phy
2.10. Cell Studies
2.10.1. hMSCs Isolation and Culture from Adipose Tissue
2.10.2. hMSCs Culture in Hydrogel Membranes
2.10.3. Cell Viability Assay
2.10.4. Cell Proliferation Assay
2.10.5. Environmental Scanning Electron Microscopy (ESEM)
2.10.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization and Viscoelastic Properties of Membranes
3.2. In Vitro Swelling and Degradation Studies
3.3. G1Phy Release
3.4. Biological Evaluation
3.4.1. ESEM Microscopy of Hydrogel Membranes
3.4.2. Cell Viability and Proliferation Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gilarska, A.; Lewandowska-Lancucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid-based injectable hydrogels for tissue engineering applications—Design, physicochemical and biological characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Remya, N.S.; Remya, S.; Nair, P.D. A biodegradable in situ injectable hydrogel based on chitosan and oxidized hyaluronic acid for tissue engineering applications. Carbohydr. Polym. 2011, 85, 838–844. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. C 2020, 114. [Google Scholar] [CrossRef]
- Suo, H.; Zhang, D.; Yin, J.; Qian, J.; Wu, Z.L.; Fu, J. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 612–620. [Google Scholar] [CrossRef]
- Abarrategi, A.; Lopiz-Morales, Y.; Ramos, V.; Civantos, A.; Lopez-Duran, L.; Marco, F.; Lopez-Lacomba, J.L. Chitosan scaffolds for osteochondral tissue regeneration. J. Biomed. Mater. Res. A 2010, 95, 1132–1141. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Greco, F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef]
- Erickson, A.E.; Sun, J.; Lan Levengood, S.K.; Swanson, S.; Chang, F.C.; Tsao, C.T.; Zhang, M. Chitosan-based composite bilayer scaffold as an in vitro osteochondral defect regeneration model. Biomed. Microdevices 2019, 21, 34. [Google Scholar] [CrossRef]
- Knudson, C.B. Hyaluronan and CD44: Strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res. Part C Embryo Today Rev. 2003, 69, 174–196. [Google Scholar] [CrossRef]
- Suri, S.; Schmidt, C.E. Photopatterned collagen-hyaluronic acid interpenetrating polymer network hydrogels. Acta Biomater. 2009, 5, 2385–2397. [Google Scholar] [CrossRef]
- Tognana, E.; Borrione, A.; De Luca, C.; Pavesio, A. Hyalograft C: Hyaluronan-based scaffolds in tissue-engineered cartilage. Cells Tissues Organs 2007, 186, 97–103. [Google Scholar] [CrossRef]
- Mohan, N.; Mohanan, P.V.; Sabareeswaran, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int. J. Biol. Macromol. 2017, 104, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, P.; Alves, P.; Valente, T.A.; Santos, R.; Correia, I.J.; Ferreira, P. Sodium hyaluronate/chitosan polyelectrolyte complex scaffolds for dental pulp regeneration: Synthesis and characterization. Int. J. Biol. Macromol. 2011, 49, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Weinstein-Oppenheimer, C.R.; Brown, D.I.; Coloma, R.; Morales, P.; Reyna-Jeldes, M.; Diaz, M.J.; Sanchez, E.; Acevedo, C.A. Design of a hybrid biomaterial for tissue engineering: Biopolymer-scaffold integrated with an autologous hydrogel carrying mesenchymal stem-cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, B.; Hu, J.; Lee, M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013, 9, 4779–4786. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, D.; Wang, Y.; Li, Y.; Li, T.; Zhou, Z.; Yang, Z.; Wang, J.; Zhang, Q. Glycol chitosan/oxidized hyaluronic acid hydrogels functionalized with cartilage extracellular matrix particles and incorporating BMSCs for cartilage repair. Artif. Cells Nanomed. Biotechnol. 2018, 46, 721–732. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.J.; Gorth, D.J.; Showalter, B.L.; Chiaro, J.A.; Beattie, E.E.; Elliott, D.M.; Mauck, R.L.; Chen, W.; Malhotra, N.R. In vitro characterization of a stem-cell-seeded triple-interpenetrating-network hydrogel for functional regeneration of the nucleus pulposus. Tissue Eng. Part A 2014, 20, 1841–1849. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, Y.; Xu, H.; Bao, Y.; Yan, X.; Li, Y.; Li, Y.; Yin, Y.; Wang, X.; Qiu, T.; et al. Preparation and evaluation of an injectable chitosan-hyaluronic acid hydrogel for peripheral nerve regeneration. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2016, 31, 1401–1407. [Google Scholar] [CrossRef]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Rubin, J.P.; Marra, K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for adipose tissue regeneration. Organogenesis 2010, 6, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Xia, C.; Mo, J.; Mei, S.; Lin, X.; Fan, S. Interpenetrating polymer network scaffold of sodium hyaluronate and sodium alginate combined with berberine for osteochondral defect regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 190–200. [Google Scholar] [CrossRef]
- Deng, C.; Chang, J.; Wu, C. Bioactive scaffolds for osteochondral regeneration. J. Orthop. Transl. 2019, 17, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Man, Y.; Li, W.; Li, L.; Xu, J.; Parungao, R.; Wang, Y.; Zheng, S.; Nie, Y.; Liu, T.; et al. 3D Bio-Printing of CS/Gel/HA/Gr Hybrid Osteochondral Scaffolds. Polymers 2019, 11, 1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragan, E.S. Advances in interpenetrating polymer network hydrogels and their applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Mora-Boza, A.; Włodarczyk-Biegun, M.K.; del Campo, A.; Vázquez-Lasa, B.; Román, J.S. Glycerylphytate as an ionic crosslinker for 3D printing of multi-layered scaffolds with improved shape fidelity and biological features. Biomater. Sci. 2020, 8, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Boza, A.; López-Donaire, M.L.; Saldaña, L.; Vilaboa, N.; Vázquez-Lasa, B.; San Román, J. Glycerylphytate compounds with tunable ion affinity and osteogenic properties. Sci. Rep. 2019, 9, 11491. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.W.; Kang, J.Y.; Yoon, I.S.; Hwang, H.D.; Balakrishnan, P.; Cho, H.J.; Chung, K.D.; Kang, D.H.; Kim, D.D. Interpenetrating polymer network (IPN) scaffolds of sodium hyaluronate and sodium alginate for chondrocyte culture. Colloids Surf. B Biointerfaces 2011, 88, 711–716. [Google Scholar] [CrossRef]
- Skaalure, S.C.; Dimson, S.O.; Pennington, A.M.; Bryant, S.J. Semi-interpenetrating networks of hyaluronic acid in degradable PEG hydrogels for cartilage tissue engineering. Acta Biomater. 2014, 10, 3409–3420. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.; Li, P.; Wang, L.; Mo, X.; Song, L.; Xu, Y.; Zhao, C.; Ouyang, B.; Tu, B.; Luo, L.; et al. An interpenetrating network-strengthened and toughened hydrogel that supports cell-based nucleus pulposus regeneration. Biomaterials 2017, 136, 12–28. [Google Scholar] [CrossRef]
- Galvez, P.; Martin, M.J.; Calpena, A.C.; Tamayo, J.A.; Ruiz, M.A.; Clares, B. Enhancing effect of glucose microspheres in the viability of human mesenchymal stem cell suspensions for clinical administration. Pharm. Res. 2014, 31, 3515–3528. [Google Scholar] [CrossRef]
- Lopez-Ruiz, E.; Jimenez, G.; Kwiatkowski, W.; Montanez, E.; Arrebola, F.; Carrillo, E.; Choe, S.; Marchal, J.A.; Peran, M. Impact of TGF-beta family-related growth factors on chondrogenic differentiation of adipose-derived stem cells isolated from lipoaspirates and infrapatellar fat pads of osteoarthritic patients. Eur. Cells Mater. 2018, 35, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yin, Y.; Yao, K.; Ma, D.; Cui, L.; Cao, Y. Influence of the concentrations of hyaluronic acid on the properties and biocompatibility of Cs-Gel-HA membranes. Biomaterials 2004, 25, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.A.; da Silva, B.C.; Riegel-Vidotti, I.C.; Urbano, A.; de Sousa Faria-Tischer, P.C.; Tischer, C.A. Production and characterization of bacterial cellulose membranes with hyaluronic acid from chicken comb. Int. J. Biol. Macromol. 2017, 97, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.D.; Peng, T.; Goosen, M.F.A.; Min, J.M.; He, Y.Y. pH-sensitivity of hydrogels based on complex forming chitosan: Polyether interpenetrating polymer network. J. Appl. Polym. Sci. 1993, 48, 343–354. [Google Scholar] [CrossRef]
- Rocha Neto, J.B.M.; Taketa, T.B.; Bataglioli, R.A.; Pimentel, S.B.; Santos, D.M.; Fiamingo, A.; Costa, C.A.R.; Campana-Filho, S.P.; Carvalho, H.F.; Beppu, M.M. Tailored chitosan/hyaluronan coatings for tumor cell adhesion: Effects of topography, charge density and surface composition. Appl. Surf. Sci. 2019, 486, 508–518. [Google Scholar] [CrossRef]
- Iacob, A.T.; Dragan, M.; Ghetu, N.; Pieptu, D.; Vasile, C.; Buron, F.; Routier, S.; Giusca, S.E.; Caruntu, I.D.; Profire, L. Preparation, Characterization and Wound Healing Effects of New Membranes Based on Chitosan, Hyaluronic Acid and Arginine Derivatives. Polymers 2018, 10, 607. [Google Scholar] [CrossRef] [Green Version]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Tamer, T.M.; Collins, M.N.; Valachova, K.; Hassan, M.A.; Omer, A.M.; Mohy-Eldin, M.S.; Svik, K.; Jurcik, R.; Ondruska, L.; Biro, C.; et al. MitoQ Loaded Chitosan-Hyaluronan Composite Membranes for Wound Healing. Materials 2018, 11, 569. [Google Scholar] [CrossRef] [Green Version]
- Noriega, S.E.; Subramanian, A. Consequences of Neutralization on the Proliferation and Cytoskeletal Organization of Chondrocytes on Chitosan-Based Matrices. Int. J. Carbohydr. Chem. 2011, 2011. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Melo, L.V.; Saramago, B.; Barbosa, M.A. Functionalization of chitosan membranes through phosphorylation: Atomic force microscopy, wettability, and cytotoxicity studies. J. Appl. Polym. Sci. 2006, 102, 276–284. [Google Scholar] [CrossRef]
- Yamanlar, S.; Sant, S.; Boudou, T.; Picart, C.; Khademhosseini, A. Surface functionalization of hyaluronic acid hydrogels by polyelectrolyte multilayer films. Biomaterials 2011, 32, 5590–5599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Liu, C.; Xia, X.; Liu, L. Conformal microcapsules encapsulating microcarrier-L02 cell complexes for treatment of acetaminophen-induced liver injury in rats. J. Mater. Chem. B 2017, 5, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Welzel, P.B.; Prokoph, S.; Zieris, A.; Grimmer, M.; Zschoche, S.; Freudenberg, U.; Werner, C. Modulating Biofunctional starPEG Heparin Hydrogels by Varying Size and Ratio of the Constituents. Polymers 2011, 3, 602–620. [Google Scholar] [CrossRef] [Green Version]
- Bean, J.E.; Alves, D.R.; Laabei, M.; Esteban, P.P.; Thet, N.T.; Enright, M.C.; Jenkins, A.T.A. Triggered Release of Bacteriophage K from Agarose/Hyaluronan Hydrogel Matrixes by Staphylococcus aureus Virulence Factors. Chem. Mater. 2014, 26, 7201–7208. [Google Scholar] [CrossRef] [Green Version]
- Correia, C.R.; Moreira-Teixeira, L.S.; Moroni, L.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; Mano, J.F. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng. Part C Methods 2011, 17, 717–730. [Google Scholar] [CrossRef] [Green Version]
- Tous, E.; Ifkovits, J.L.; Koomalsingh, K.J.; Shuto, T.; Soeda, T.; Kondo, N.; Gorman, J.H., 3rd; Gorman, R.C.; Burdick, J.A. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules 2011, 12, 4127–4135. [Google Scholar] [CrossRef] [Green Version]
- Barahuie, F.; Dorniani, D.; Saifullah, B.; Gothai, S.; Hussein, M.Z.; Pandurangan, A.K.; Arulselvan, P.; Norhaizan, M.E. Sustained release of anticancer agent phytic acid from its chitosan-coated magnetic nanoparticles for drug-delivery system. Int. J. Nanomed. 2017, 12, 2361–2372. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-Y.; Hsu, C.-H.; Tsai, M.-L. Effect of crosslinked condition on characteristics of chitosan/tripolyphosphate/genipin beads and their application in the selective adsorption of phytic acid from soybean whey. Carbohydr. Polym. 2011, 86, 659–665. [Google Scholar] [CrossRef]
- Ayala, R.; Zhang, C.; Yang, D.; Hwang, Y.; Aung, A.; Shroff, S.S.; Arce, F.T.; Lal, R.; Arya, G.; Varghese, S. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011, 32, 3700–3711. [Google Scholar] [CrossRef]
- Barlian, A.; Judawisastra, H.; Alfarafisa, N.M.; Wibowo, U.A.; Rosadi, I. Chondrogenic differentiation of adipose-derived mesenchymal stem cells induced by L-ascorbic acid and platelet rich plasma on silk fibroin scaffold. PeerJ 2018, 6, e5809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Wu, Y.; Li, C.; Zhang, T.; Zou, Y.; Hui, J.H.; Ge, Z.; Lee, E.H. Improved mesenchymal stem cells attachment and in vitro cartilage tissue formation on chitosan-modified poly(L-lactide-co-epsilon-caprolactone) scaffold. Tissue Eng. Part A 2012, 18, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Boza, A.; García-Fernández, L.; Barbosa, F.A.; Oliveira, A.L.; Vázquez-Lasa, B.; San Román, J. Glycerylphytate crosslinker as a potential osteoinductor of chitosan-based systems for guided bone regeneration. Carbohydr. Polym. 2020, 241, 116269. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; van Weeren, P.R.; Dhert, W.J.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef]







| Membrane Sample | C a | H a | N a | C/N a | G1Phy Content (%) b,c | Yield (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Theo | Exp | Theo | Exp | Theo | Exp | Theo | Exp | |||
| Ch | 44.9 | 43.03 ± 0.33 | 6.80 | 6.86 ± 0.01 | 8.60 | 8.24 ± 0.10 | 5.2 | 5.22 ± 0.02 | 5.4 ± 0.1 | 95.3 ± 1.1 |
| Ch/HA | 44.7 | 42.72 ± 0.11 | 6.53 | 6.77 ± 0.09 | 7.36 | 8.12 ± 0.11 | 6.1 | 5.25 ± 0.07 | 4.9 ± 0.5 | 75.0 ± 0.6 |
| Ch/HAMA | 44.7 | 42.11 ± 0.18 | 6.53 | 6.58 ± 0.15 | 7.36 | 7.21 ± 0.19 | 6.1 | 5.83 ± 0.15 | 2.5 ± 0.4 | 93.5 ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Boza, A.; López-Ruiz, E.; López-Donaire, M.L.; Jiménez, G.; Aguilar, M.R.; Marchal, J.A.; Pedraz, J.L.; Vázquez-Lasa, B.; Román, J.S.; Gálvez-Martín, P. Evaluation of Glycerylphytate Crosslinked Semi- and Interpenetrated Polymer Membranes of Hyaluronic Acid and Chitosan for Tissue Engineering. Polymers 2020, 12, 2661. https://doi.org/10.3390/polym12112661
Mora-Boza A, López-Ruiz E, López-Donaire ML, Jiménez G, Aguilar MR, Marchal JA, Pedraz JL, Vázquez-Lasa B, Román JS, Gálvez-Martín P. Evaluation of Glycerylphytate Crosslinked Semi- and Interpenetrated Polymer Membranes of Hyaluronic Acid and Chitosan for Tissue Engineering. Polymers. 2020; 12(11):2661. https://doi.org/10.3390/polym12112661
Chicago/Turabian StyleMora-Boza, Ana, Elena López-Ruiz, María Luisa López-Donaire, Gema Jiménez, María Rosa Aguilar, Juan Antonio Marchal, José Luis Pedraz, Blanca Vázquez-Lasa, Julio San Román, and Patricia Gálvez-Martín. 2020. "Evaluation of Glycerylphytate Crosslinked Semi- and Interpenetrated Polymer Membranes of Hyaluronic Acid and Chitosan for Tissue Engineering" Polymers 12, no. 11: 2661. https://doi.org/10.3390/polym12112661
APA StyleMora-Boza, A., López-Ruiz, E., López-Donaire, M. L., Jiménez, G., Aguilar, M. R., Marchal, J. A., Pedraz, J. L., Vázquez-Lasa, B., Román, J. S., & Gálvez-Martín, P. (2020). Evaluation of Glycerylphytate Crosslinked Semi- and Interpenetrated Polymer Membranes of Hyaluronic Acid and Chitosan for Tissue Engineering. Polymers, 12(11), 2661. https://doi.org/10.3390/polym12112661