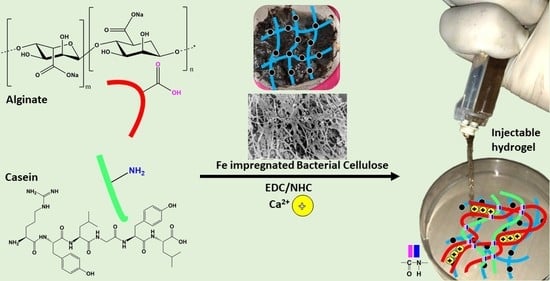
Effect of Iron-Oxide Nanoparticles Impregnated Bacterial Cellulose on Overall Properties of Alginate/Casein Hydrogels: Potential Injectable Biomaterial for Wound Healing Applications
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Materials
2.2. Synthesis of Iron-Oxide Nanoparticles (FeNPs)
2.3. Synthesis of Bacterial Cellulose Impregnated with Iron-Oxide Nanoparticles (BCF)
2.4. Preparation of Water Soluble Casein.
2.5. Synthesis of BCF Loaded Alginate/Casein Injectable Hydrogels
2.6. Instrumentation and Characterization Methodology
2.6.1. Fourier Transformed Infrared Spectroscopy
2.6.2. X-ray Diffraction Studies
2.6.3. Morphological and Elemental Analysis
2.6.4. Mechanical Compression Analysis
2.6.5. Swelling Properties
2.6.6. Thermogravimetric Analysis
2.6.7. Viscoelastic Properties
2.6.8. Differential Scanning Calorimetry
2.6.9. Drug Release Studies
2.6.10. Antibacterial Activity
2.6.11. In-Vitro Cytocompatibility Studies
3. Results and Discussion
3.1. Synthesis of Iron-Oxide Nanoparticles (FeNPs) and Bacterial Cellulose Impregnated with Iron-Oxide Nanoparticles (BCF)
3.2. Morphological Analysis of Alginate–Casein-FeNP-Impregnated Bacterial Cellulose (ACB) Hydrogels
3.3. Swelling Properties of Alginate–Casein-FeNP-Impregnated Bacterial Cellulose (ACB) Hydrogels
3.4. Infrared Spectroscopy Studies of Alginate–Casein-FeNP-Impregnated Bacterial Cellulose (ACB) Hydrogels
3.5. X-Ray Diffraction Analysis of Alginate–Casein-FeNP-Impregnated Bacterial Cellulose (ACB) Hydrogels
3.6. Compression Analysis of Prepared Alginate–Casein-FeNP-Impregnated Bacterial Cellulose (ACB) Hydrogels
3.7. Viscoelastic Behavior of Alginate–Casein-FeNP-Impregnated Bacterial Cellulose (ACB) Hydrogels
3.8. Thermal Property Analysis of Prepared Alginate–Casein-FeNP-Impregnated Bacterial Cellulose (ACB) Hydrogels
3.9. Magnetic Properties of Prepared Alginate–Casein-FeNP-Impregnated Bacterial Cellulose (ACB) Hydrogels
3.10. Drug Release Profiles of Prepared Alginate–Casein-FeNP-Impregnated Bacterial Cellulose (ACB) Hydrogels
3.11. Antibacterial and Cytotoxicity Analysis of Prepared Alginate–Casein-FeNP-Impregnated Bacterial Cellulose (ACB) Hydrogels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patwa, R.; Soundararajan, N.; Mulchandani, N.; Bhasney, S.M.; Shah, M.; Kumar, S.; Kumar, A.; Katiyar, V. Silk nano-discs: A natural material for cancer therapy. Biopolymers 2018, 109, e23231. [Google Scholar] [CrossRef]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in Medical Implants: A Brief Review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jeon, S.; Yang, J.; Baek, S.Y.; Kim, D. Hydrogel Nanoparticle as a Functional Coating Layer in Biosensing, Tissue Engineering, and Drug Delivery. Coatings 2020, 10, 663. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Moreira, H.R.; Munarin, F.; Gentilini, R.; Visai, L.; Granja, P.L.; Tanzi, M.C.; Petrini, P. Injectable pectin hydrogels produced by internal gelation: pH dependence of gelling and rheological properties. Carbohydr. Polym. 2014, 103, 339–347. [Google Scholar] [CrossRef]
- Lv, X.; Liu, Y.; Song, S.; Tong, C.; Shi, X.; Zhao, Y.; Zhang, J.; Hou, M. Influence of chitosan oligosaccharide on the gelling and wound healing properties of injectable hydrogels based on carboxymethyl chitosan/alginate polyelectrolyte complexes. Carbohydr. Polym. 2019, 205, 312–321. [Google Scholar] [CrossRef]
- Resmi, R.; Parvathy, J.; John, A.; Joseph, R.; Rajalekshmi, R.; Jayasree, P.; Annie, J.; Roy, J. Injectable self-crosslinking hydrogels for meniscal repair: A study with oxidized alginate and gelatin. Carbohydr. Polym. 2020, 234, 115902. [Google Scholar] [CrossRef]
- Mehrotra, D.; Dwivedi, R.; Nandana, D.; Singh, R. From injectable to 3D printed hydrogels in maxillofacial tissue engineering: A review. J. Oral Biol. Craniofacial Res. 2020, 10, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Wu, Y.; Wang, H.; Liu, J.; Ma, Q.; Xiao, K.; Li, Z.; Li, J.; Luo, F.; Tan, H. An injectable hydrogel with pH-sensitive and self-healing properties based on 4armPEGDA and N-carboxyethyl chitosan for local treatment of hepatocellular carcinoma. Int. J. Biol. Macromol. 2020, 163, 1208–1222. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Dahiya, U.R.; Yadav, S.; Sharma, P.; Ghosh, D.; Rao, G.K.; Rawat, V.; Kumar, G.; Kumar, A.; Srivastava, C. Zinc Oxide Nanoparticles Functionalized on Hydrogel Grafted Silk Fibroin Fabrics as Efficient Composite Dressing. Biomolecules 2020, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Patwa, R.; Saha, N.; Sáha, P. Magnetic hydrogel based shoe insoles for prevention of diabetic foot. J. Magn. Magn. Mater. 2020, 514, 167153. [Google Scholar] [CrossRef]
- Li, B.; Wu, C.; Wang, C.; Luo, Z.; Cao, J. Fabrication of tough, self-recoverable, and electrically conductive hydrogels by in situ reduction of poly(acrylic acid) grafted graphene oxide in polyacrylamide hydrogel matrix. J. Appl. Polym. Sci. 2019, 137, 48781. [Google Scholar] [CrossRef]
- Barczak, M.; Borowski, P.; Gila-Vilchez, C.; Alaminos, M.; González-Caballero, F.; López-López, M.T. Revealing importance of particles’ surface functionalization on the properties of magnetic alginate hydrogels. Carbohydr. Polym. 2020, 247, 116747. [Google Scholar] [CrossRef]
- Li, D.-Q.; Wang, S.-Y.; Meng, Y.-J.; Li, J.-F.; Li, J. An injectable, self-healing hydrogel system from oxidized pectin/chitosan/γ-Fe2O3. Int. J. Biol. Macromol. 2020, 164, 4566–4574. [Google Scholar] [CrossRef]
- Yan, E.; Cao, M.; Ren, X.; Jiang, J.; An, Q.; Zhang, Z.; Gao, J.; Yang, X.; Zhang, D. Synthesis of Fe3O4 nanoparticles functionalized polyvinyl alcohol/chitosan magnetic composite hydrogel as an efficient adsorbent for chromium (VI) removal. J. Phys. Chem. Solids 2018, 121, 102–109. [Google Scholar] [CrossRef]
- Patwa, R.; Saha, N.; Saha, P. Magnetic hydrogel based shoe insoles for diabetics. In Proceedings of the 35th International Conference of the Polymer Processing Society (PPS-35), Cesme-Izmir, Turkey, 26–30 May 2019; AIP Publishing: Melville, NY, USA, 2020; Volume 2205, p. 020027. [Google Scholar]
- Guo, J.; Filpponen, I.; Johansson, L.-S.; Mohammadi, P.; Latikka, M.; Linder, M.B.; Ras, R.H.A.; Rojas, O.J. Complexes of Magnetic Nanoparticles with Cellulose Nanocrystals as Regenerable, Highly Efficient, and Selective Platform for Protein Separation. Biomacromolecules 2017, 18, 898–905. [Google Scholar] [CrossRef]
- Arias, S.L.; Shetty, A.R.; Senpan, A.; Echeverry-Rendón, M.; Reece, L.M.; Allain, J.P. Fabrication of a Functionalized Magnetic Bacterial Nanocellulose with Iron Oxide Nanoparticles. J. Vis. Exp. 2016, 111, e52951. [Google Scholar] [CrossRef] [Green Version]
- Patwa, R.; Saha, N.; Saha, P.; Katiyar, V. Biocomposites of poly(lactic acid) and lactic acid oligomer-grafted bacterial cellulose: It’s preparation and characterization. J. Appl. Polym. Sci. 2019, 136, 47903. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Jooyeon, P.; Cho, S.-P.; Sung, D.; Ryu, S.; Park, M.; Min, K.-A.; Kim, J.; Hong, S.; et al. In situ hybridization of carbon nanotubes with bacterial cellulose for three-dimensional hybrid bioscaffolds. Biomaterials 2015, 58, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Etula, J.; Bankar, S. In Situ Bioprocessing of Bacterial Cellulose with Graphene: Percolation Network Formation, Kinetic Analysis with Physicochemical and Structural Properties Assessment. ACS Appl. Bio Mater. 2019, 2, 4052–4066. [Google Scholar] [CrossRef] [Green Version]
- Serafica, G.; Mormino, R.; Bungay, H. Inclusion of solid particles in bacterial cellulose. Appl. Microbiol. Biotechnol. 2002, 58, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Galateanu, B.; Bunea, M.-C.; Stanescu, P.; Vasile, E.; Casarica, A.; Iovu, H.; Hermenean, A.; Zaharia, C.; Costache, M. In Vitro Studies of Bacterial Cellulose and Magnetic Nanoparticles Smart Nanocomposites for Efficient Chronic Wounds Healing. Stem Cells Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jaikumar, D.; Sajesh, K.; Soumya, S.; Nimal, T.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Injectable alginate-O-carboxymethyl chitosan/nano fibrin composite hydrogels for adipose tissue engineering. Int. J. Biol. Macromol. 2015, 74, 318–326. [Google Scholar] [CrossRef]
- Stilhano, R.S.; Madrigal, J.L.; Wong, K.; Williams, P.A.; Martin, P.K.; Yamaguchi, F.S.; Samoto, V.Y.; Han, S.W.; Silva, E.A. Injectable alginate hydrogel for enhanced spatiotemporal control of lentivector delivery in murine skeletal muscle. J. Control. Release 2016, 237, 42–49. [Google Scholar] [CrossRef]
- Wang, K.; Nune, K.; Misra, R. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef]
- Li, N.-N.; Fu, C.-P.; Zhang, L.-M. Using casein and oxidized hyaluronic acid to form biocompatible composite hydrogels for controlled drug release. Mater. Sci. Eng. C 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Zhang, Z.; Decker, E.A.; McClements, D.J. Encapsulation, protection, and release of polyunsaturated lipids using biopolymer-based hydrogel particles. Food Res. Int. 2014, 64, 520–526. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Z.; Zhang, R.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Encapsulation of protein nanoparticles within alginate microparticles: Impact of pH and ionic strength on functional performance. J. Food Eng. 2016, 178, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, S.K.; Shah, F.F.; Bajpai, M. Dynamic release of gentamicin sulfate (GS) from alginate dialdehyde (AD)-crosslinked casein (CAS) films for antimicrobial applications. Des. Monomers Polym. 2016, 20, 18–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, P.; Kumar, A.; Katiyar, V. Magnetic Cellulose Nanocrystal Based Anisotropic Polylactic Acid Nanocomposite Films: Influence on Electrical, Magnetic, Thermal, and Mechanical Properties. ACS Appl. Mater. Interfaces 2016, 8, 18393–18409. [Google Scholar] [CrossRef] [PubMed]
- Mazrouaa, A.M.; Mohamed, M.G.; Fekry, M. Physical and magnetic properties of iron oxide nanoparticles with a different molar ratio of ferrous and ferric. Egypt. J. Pet. 2019, 28, 165–171. [Google Scholar] [CrossRef]
- Khramtsov, P.; Barkina, I.; Kropaneva, M.; Bochkova, M.; Timganova, V.; Nechaev, A.; Byzov, I.; Zamorina, S.; Yermakov, A.; Rayev, M. Magnetic Nanoclusters Coated with Albumin, Casein, and Gelatin: Size Tuning, Relaxivity, Stability, Protein Corona, and Application in Nuclear Magnetic Resonance Immunoassay. Nanomaterials 2019, 9, 1345. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.J.B.; Neves, M.C.; Neto, C.P.; Trindade, T. Growth and Chemical Stability of Copper Nanostructures on Cellulosic Fibers. Eur. J. Inorg. Chem. 2012, 2012, 5043–5049. [Google Scholar] [CrossRef]
- Pinto, R.J.; Marques, P.A.A.P.; Martins, M.A.; Neto, C.P.; Trindade, T. Electrostatic assembly and growth of gold nanoparticles in cellulosic fibres. J. Colloid Interface Sci. 2007, 312, 506–512. [Google Scholar] [CrossRef]
- Taokaew, S.; Seetabhawang, S.; Siripong, P.; Phisalaphong, M. Biosynthesis and Characterization of Nanocellulose-Gelatin Films. Materials 2013, 6, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Chunshom, N.; Chuysinuan, P.; Techasakul, S.; Ummartyotin, S. Dried-state bacterial cellulose (Acetobacter xylinum) and polyvinyl-alcohol-based hydrogel: An approach to a personal care material. J. Sci. Adv. Mater. Devices 2018, 3, 296–302. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, S.; Zhong, L.; Wang, B.; Wang, H.; Hong, F.F. Zn2+-loaded TOBC nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 143, 235–242. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Igarzabal, C.I.A. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kabutare, Y.H.; Ghosh, P. Dual crosslinked keratin-alginate fibers formed via ionic complexation of amide networks with improved toughness for assembling into braids. Polym. Test. 2020, 81, 106286. [Google Scholar] [CrossRef]
- Jabeen, S.; Chat, O.A.; Maswal, M.; Ashraf, U.; Rather, G.M.; Dar, A.A. Hydrogels of sodium alginate in cationic surfactants: Surfactant dependent modulation of encapsulation/release toward Ibuprofen. Carbohydr. Polym. 2015, 133, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Hartrianti, P.; Nguyen, L.T.H.; Johanes, J.; Chou, S.M.; Zhu, P.; Tan, N.S.; Tang, M.B.Y.; Ng, K.W. Fabrication and characterization of a novel crosslinked human keratin-alginate sponge. J. Tissue Eng. Regen. Med. 2017, 11, 2590–2602. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-alginate hydrogel for cell encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.M.; Aguilera, J.M.; Park, D.J. Mechanical, rheological and structural properties of fiber-containing microgels based on whey protein and alginate. Carbohydr. Polym. 2019, 207, 571–579. [Google Scholar] [CrossRef]
- Dávila, J.L.; D’Ávila, M.A. Rheological evaluation of Laponite/alginate inks for 3D extrusion-based printing. Int. J. Adv. Manuf. Technol. 2018, 101, 675–686. [Google Scholar] [CrossRef]
- Rescignano, N.; Hernández, R.; Lopez, L.D.; Calvillo, I.; Kenny, J.M.; Mijangos, C. Preparation of alginate hydrogels containing silver nanoparticles: A facile approach for antibacterial applications. Polym. Int. 2016, 65, 921–926. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, Y.; Wang, G.; Lin, Q.; Fan, J. pH- and electro-response characteristics of bacterial cellulose nanofiber/sodium alginate hybrid hydrogels for dual controlled drug delivery. RSC Adv. 2014, 4, 47056–47065. [Google Scholar] [CrossRef]
- Siročić, A.P.; Krehula, L.K.; Katančić, Z.; Hrnjak-Murgić, Z. Characterization of Casein Fractions—Comparison of Commercial Casein and Casein Extracted from Cow’s Milk. Chem. Biochem. Eng. Q. 2017, 30, 501–509. [Google Scholar] [CrossRef]
- Sulaeva, I.; Hettegger, H.; Bergen, A.; Rohrer, C.; Kostic, M.; Konnerth, J.; Rosenau, T.; Potthast, A. Fabrication of bacterial cellulose-based wound dressings with improved performance by impregnation with alginate. Mater. Sci. Eng. C 2020, 110, 110619. [Google Scholar] [CrossRef] [PubMed]











| Sample | Alginate (% w/v) | Casein (% w/v) | BCF (% w/w) |
|---|---|---|---|
| ACB0 | 2 | 2 | - |
| ACB12.5 | 2 | 2 | 12.5 |
| ACB25 | 2 | 2 | 25 |
| ACB50 | 2 | 2 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patwa, R.; Zandraa, O.; Capáková, Z.; Saha, N.; Sáha, P. Effect of Iron-Oxide Nanoparticles Impregnated Bacterial Cellulose on Overall Properties of Alginate/Casein Hydrogels: Potential Injectable Biomaterial for Wound Healing Applications. Polymers 2020, 12, 2690. https://doi.org/10.3390/polym12112690
Patwa R, Zandraa O, Capáková Z, Saha N, Sáha P. Effect of Iron-Oxide Nanoparticles Impregnated Bacterial Cellulose on Overall Properties of Alginate/Casein Hydrogels: Potential Injectable Biomaterial for Wound Healing Applications. Polymers. 2020; 12(11):2690. https://doi.org/10.3390/polym12112690
Chicago/Turabian StylePatwa, Rahul, Oyunchimeg Zandraa, Zdenka Capáková, Nabanita Saha, and Petr Sáha. 2020. "Effect of Iron-Oxide Nanoparticles Impregnated Bacterial Cellulose on Overall Properties of Alginate/Casein Hydrogels: Potential Injectable Biomaterial for Wound Healing Applications" Polymers 12, no. 11: 2690. https://doi.org/10.3390/polym12112690
APA StylePatwa, R., Zandraa, O., Capáková, Z., Saha, N., & Sáha, P. (2020). Effect of Iron-Oxide Nanoparticles Impregnated Bacterial Cellulose on Overall Properties of Alginate/Casein Hydrogels: Potential Injectable Biomaterial for Wound Healing Applications. Polymers, 12(11), 2690. https://doi.org/10.3390/polym12112690