Efficient and Low Cytotoxicity Gene Carriers Based on Amine-Functionalized Polyvinylpyrrolidone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Polyvinylpyrrolidone Backbone Polymers
2.2. Preparation and Characterization of Polymer/Plasmid Complexes
2.3. In Vitro Cellular Studies
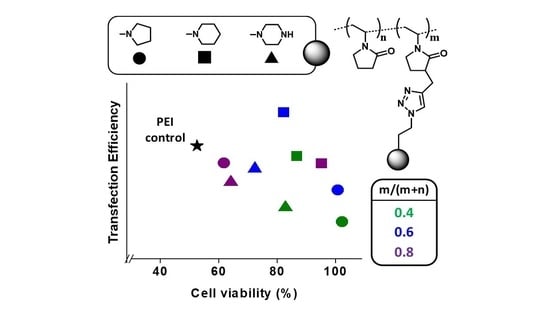
3. Results and Discussion
3.1. Polyplexes Analysis
3.2. Cell Culture Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verma, I.M.; Somia, N. Gene therapy—Promises, problems and prospects. Nature 1997, 389, 239–242. [Google Scholar] [CrossRef]
- Khan, W.; Hosseinkhani, H.; Ickowicz, D.; Hong, P.D.; Yu, D.-S.; Domb, A.J. Polysaccharide gene transfection agents. Acta Biomater. 2012, 8, 4224–4232. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Glover, D.-J.; Lipps, H.-J.; Jans, D.-A. Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev. Genet. 2005, 6, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral vectors: A look back and ahead on gene transfer technology. New Microbiol. 2013, 36, 1–22. [Google Scholar]
- Hosseinkhani, H.; Abedini, F.; Ou, K.-L.; Domb, A.-J. Polymers in gene therapy technology. Polym. Adv. Technol. 2015, 26, 198–211. [Google Scholar] [CrossRef]
- Lai, W.-F.; Wong, W.-T. Design of polymeric gene carriers for effective intracellular delivery. Trends Biotechnol. 2018, 36, 713–728. [Google Scholar] [CrossRef]
- Alibolandi, M.; Abnous, K.; Sadeghi, F.; Hosseinkhani, H.; Ramezani, M.; Hadizadeh, F. Folate receptor-targeted multimodal polymersomes for delivery of quantum dots and doxorubicin to breast adenocarcinoma: In vitro and in vivo evaluation. Int. J. Pharm. 2016, 500, 162–178. [Google Scholar] [CrossRef]
- Abedini, F.; Hosseinkhani, H.; Ismail, M.; Domb, A.-J.; Omar, A.R.; Chong, P.P.; Hong, P.-D.; Yu, D.-S.; Farber, I.-Y. Cationized dextran nanoparticle-encapsulated CXCR4-siRNA enhanced correlation between CXCR4 expression and serum alkaline phosphatase in a mouse model of colorectal cancer. Int. J. Nanomed. 2012, 7, 4159–4168. [Google Scholar]
- Ghadiri, M.; Vasheghani-Farahani, E.; Atyabi, F.; Kobarfard, F.; Mohamadyar-Toupkanlou, F.; Hosseinkhani, H. Transferrin-conjugated magnetic dextran-spermine nanoparticles for targeted drug transport across blood-brain barrier. J. Biomed. Mater. Res. A 2017, 105, 2851–2864. [Google Scholar] [CrossRef]
- Barua, S.; Ramos, J.; Potta, T.; Taylor, D.; Huang, H.-C.; Montanez, G.; Rege, K. Discovery of cationic polymers for non-viral gene delivery using combinatorial approaches. Comb. Chem. High Throughput Screen. 2011, 14, 908–924. [Google Scholar] [CrossRef] [Green Version]
- Wolfert, M.-A.; Dash, P.-R.; Nazarova, O.; Oupicky, D.; Seymour, L.-W.; Smart, S.; Strohalm, J.; Ulbrich, K. Polyelectrolyte vectors fo gene delivery: Influence of cationic polymer on biophysical properties of complexes formed with DNA. Bioconjug. Chem. 1999, 10, 993–1004. [Google Scholar] [CrossRef]
- Wu, G.-Y.; Wu, C.-H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 1987, 262, 4429–4432. [Google Scholar]
- Lim, Y.-B.; Kim, C.-H.; Kim, K.; Kim, S.-W.; Park, J.-S. Development of a safe gene delivery system using biodegradable polymer, poly [α-(4-aminobutyl)-L-glycolic acid]. J. Am. Chem. Soc. 2000, 122, 6524–6525. [Google Scholar] [CrossRef]
- Bose, R.-J.; Lee, S.-H.; Park, H. Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. Biomater. Res. 2016, 20, 34. [Google Scholar] [CrossRef] [Green Version]
- Wightman, L.; Kircheis, R.; Rössler, V.; Carotta, S.; Ruzicka, R.; Kursa, M.; Wagner, E. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J. Gene Med. 2001, 3, 362–372. [Google Scholar] [CrossRef]
- Oupicky, D.; Ogris, M.; Howard, K.-A.; Dash, P.-R.; Ulbrich, K.; Seymour, L.-W. Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol. Ther. 2002, 5, 463–472. [Google Scholar] [CrossRef]
- Choosakoonkriang, S.; Lobo, B.-A.; Koe, G.-S.; Koe, J.-G.; Middaugh, C.-R. Biophysical characterization of PEI/DNA complexes. J. Pharm. Sci. 2003, 92, 1710–1722. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Mao, H.; Leong, K. Enhanced gene expression in mouse muscle by sustained release of plasmid DNA using PPE-EA as a carrier. Gene Ther. 2002, 9, 1254–1261. [Google Scholar] [CrossRef] [Green Version]
- Haensler, J.; Szoka, F.-C., Jr. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug. Chem. 1993, 4, 372–379. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.-A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhuo, R.-X.; Cheng, S.-X. Alginate modified nanostructured calcium carbonate with enhanced delivery efficiency for gene and drug delivery. Mol. Biosyst. 2012, 8, 753–759. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, F.-C.; Mumper, R.-J.; Wang, J.; Tagliaferri, J.-M.; Gill, I.; Hinchcliffe, M.; Rolland, A.-P. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J. Control. Release 1998, 56, 259–272. [Google Scholar] [CrossRef]
- Lee, M.; Nah, J.-W.; Kwon, Y.; Koh, J.-J.; Ko, K.-S.; Kim, S.-W. Water-soluble and low molecular weight chitosan-based plasmid DNA delivery. Pharm. Res. 2001, 18, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, N.-D.; Szoka, F.-C.; Verkman, A.-S. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J. Biol. Chem. 2003, 278, 44826–44831. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Klibanov, A.-M. Enhancing polyethylenimine’s delivery of plasmid DNA into mammalian cells. Proc. Natl. Acad. Sci. USA 2002, 99, 14640–14645. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.-H.; Chae, S.-Y.; Bae, Y.-H.; Kim, S.-W. Biodegradable poly (ethylenimine) for plasmid DNA delivery. J. Control. Release 2002, 80, 273–282. [Google Scholar] [CrossRef]
- Putnam, D.; Gentry, C.-A.; Pack, D.-W.; Langer, R. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc. Natl. Acad. Sci. USA 2001, 98, 1200–1205. [Google Scholar] [CrossRef]
- Mishra, S.; Heidel, J.-D.; Webster, P.; Davis, M.-E. Imidazole groups on a linear, cyclodextrin-containing polycation produce enhanced gene delivery via multiple processes. J. Control. Release 2006, 116, 179–191. [Google Scholar] [CrossRef]
- Pun, S.-H.; Bellocq, N.-C.; Liu, A.; Jensen, G.; Machemer, T.; Quijano, E.; Schluep, T.; Wen, S.; Engler, H.; Heidel, J. Cyclodextrin-modified polyethylenimine polymers for gene delivery. Bioconjug. Chem. 2004, 15, 831–840. [Google Scholar] [CrossRef]
- Redondo, J.-A.; Martínez-Campos, E.; Plet, L.; Pérez-Perrino, M.; Navarro, R.; Corrales, G.; Pandit, A.; Reinecke, H.; Gallardo, A.; López-Lacomba, J.-L.; et al. Polymeric gene carriers bearing pendant β-cyclodextrin: The relevance of glycoside permethylation on the “in vitro” cell response. Macromol. Rapid Commun. 2016, 37, 575–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumper, R.-J.; Duguid, J.-G.; Anwer, K.; Barron, M.-K.; Nitta, H.; Rolland, A.-P. Polyvinyl derivatives as novel interactive polymers for controlled gene delivery to muscle. Pharm. Res. 1996, 13, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, Y.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Yamamoto, Y.; Kodaira, H.; Tsunoda, S.; Okamoto, T.; Mukai, Y.; Shibata, H.; et al. The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials 2004, 25, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.-J.; Chou, L.-C.; Bee, Y.-S.; Chen, J.-F.; Lin, H.-C.; Lin, P.-R.; Lam, H.-C.; Tai, M.-H. Suppression of choroidal neovascularization by intramuscular polymer-based gene delivery of vasostatin. Exp. Eye Res. 2005, 81, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qiao, H.; Jiang, H.; Zhi, X.; Liu, F.; Wang, J.; Liu, M.; Krissansen, G.-W. Intramuscular delivery of antiangiogenic genes suppresses secondary metastases after removal of primary tumors. Cancer Gene Ther. 2005, 12, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.-E.; Park, I.-K.; Kim, E.-M.; Jeong, H.-J.; Park, T.-G.; Choi, Y.-J.; Akaike, T.; Cho, C.-S. Intramuscular delivery of antiangiogenic genes suppresses secondary metastases after removal of primary tumors. J. Control. Release 2005, 105, 151–163. [Google Scholar] [CrossRef]
- Lim, D.-W.; Yeom, Y.-I.; Park, T.-G. Poly (DMAEMA-NVP)-b-PEG-galactose as gene delivery vector for hepatocytes. Bioconjug. Chem. 2000, 11, 688–695. [Google Scholar] [CrossRef]
- Zhong, Z.; Song, Y.; Engbersen, J.-F.; Lok, M.-C.; Hennink, W.-E.; Feijen, J. A versatile family of degradable non-viral gene carriers based on hyperbranched poly (ester amine) s. J. Control. Release 2005, 109, 317–329. [Google Scholar] [CrossRef]
- Velasco, D.; Collin, E.; San Roman, J.; Pandit, A.; Elvira, C. End functionalized polymeric system derived from pyrrolidine provide high transfection efficiency. Eur. J. Pharm. Biopharm. 2011, 79, 485–494. [Google Scholar] [CrossRef]
- Velasco, D.; Réthoré, G.; Newland, B.; Parra, J.; Elvira, C.; Pandit, A.; Rojo, L.; San Román, J. Low polydispersity (N-ethyl pyrrolidine methacrylamide-co-1-vinylimidazole) linear oligomers for gene therapy applications. Eur. J. Pharm. Biopharm. 2012, 82, 465–474. [Google Scholar] [CrossRef]
- Li, R.-Q.; Niu, Y.-L.; Zhao, N.-N.; Yu, B.-R.; Mao, C.; Xu, F.-J. Series of new β-cyclodextrin-cored starlike carriers for gene delivery. ACS Appl. Mater. Interfaces 2014, 6, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Redondo, J.-A.; Velasco, D.; Pérez-Perrino, M.; Reinecke, H.; Gallardo, A.; Pandit, A.; Elvira, C. Synergistic effect of pendant hydroxypropyl and pyrrolidine moieties randomly distributed along polymethacrylamide backbones on in vitro DNA-transfection. Eur. J. Pharm. Biopharm. 2015, 90, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Prado, A.; Navarro, R.; Levkin, P.-A.; Gallardo, A.; Elvira, C.; Reinecke, H. Dual stimuli-responsive polyamines derived from modified N-vinylpyrrolidones through CuAAC click chemistry. J. Polym. Sci. Pol. Chem. 2016, 54, 1098–1108. [Google Scholar] [CrossRef]








| FVPcpl | fVPcpla | Mn (kDa) b | Đb |
| 0.20 | 0.18 | 46.2 | 1.54 |
| 0.40 | 0.43 | 46.4 | 1.65 |
| 0.60 | 0.54 | 44.0 | 1.75 |
| 0.80 | 0.78 | 41.9 | 1.78 |
| 1.00 | 1.00 | 33.4 | 1.72 |
| FVPcpp | fVPcppa | Mn (kDa) b | Đb |
| 0.20 | 0.17 | 42.9 | 1.46 |
| 0.40 | 0.44 | 39.2 | 1.44 |
| 0.60 | 0.60 | 36.7 | 1.49 |
| 0.80 | 0.83 | 28.2 | 1.52 |
| 1.00 | 1.00 | 23.2 | 1.51 |
| FVPcpz-Boc * | fVPcpz-Boca,* | Mn (kDa) b,* | Đb,* |
| 0.20 | 0.19 | 41.9 | 2.10 |
| 0.40 | 0.36 | 42.3 | 2.15 |
| 0.60 | 0.58 | 45.7 | 1.94 |
| 0.80 | 0.78 | 48.3 | 1.82 |
| 1.00 | 1.00 | 63.3 | 1.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Prado, A.; Civantos, A.; Martínez-Campos, E.; Levkin, P.A.; Reinecke, H.; Gallardo, A.; Elvira, C. Efficient and Low Cytotoxicity Gene Carriers Based on Amine-Functionalized Polyvinylpyrrolidone. Polymers 2020, 12, 2724. https://doi.org/10.3390/polym12112724
Del Prado A, Civantos A, Martínez-Campos E, Levkin PA, Reinecke H, Gallardo A, Elvira C. Efficient and Low Cytotoxicity Gene Carriers Based on Amine-Functionalized Polyvinylpyrrolidone. Polymers. 2020; 12(11):2724. https://doi.org/10.3390/polym12112724
Chicago/Turabian StyleDel Prado, Anselmo, Ana Civantos, Enrique Martínez-Campos, Pavel A. Levkin, Helmut Reinecke, Alberto Gallardo, and Carlos Elvira. 2020. "Efficient and Low Cytotoxicity Gene Carriers Based on Amine-Functionalized Polyvinylpyrrolidone" Polymers 12, no. 11: 2724. https://doi.org/10.3390/polym12112724
APA StyleDel Prado, A., Civantos, A., Martínez-Campos, E., Levkin, P. A., Reinecke, H., Gallardo, A., & Elvira, C. (2020). Efficient and Low Cytotoxicity Gene Carriers Based on Amine-Functionalized Polyvinylpyrrolidone. Polymers, 12(11), 2724. https://doi.org/10.3390/polym12112724