Single and Double-Stranded 1D-Coordination Polymers with 4′-(4-Alkyloxyphenyl)-3,2′:6′,3″-terpyridines and {Cu2(μ-OAc)4} or {Cu4(μ3-OH)2(μ-OAc)2(μ3-OAc)2(AcO-κO)2} Motifs
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Compound 3
2.3. Compound 4
2.4. Reaction between 1 and Cu(OAc)2·H2O: Experiment 1
2.5. Reaction between 1 and Cu(OAc)2·H2O: Experiment 2
2.6. Preparative Scale Reaction between 1 and Cu(OAc)2·H2O to Give [Cu2(OAc)4(1)]n
2.7. Crystal Growth of [2{Cu2(μ-OAc)4(2)}·1.25MeOH]n
2.8. Crystal Growth of [Cu2(μ-OAc)4(3)]n
2.9. Crystal Growth of [{Cu2(μ-OAc)4(4)}·0.2CHCl3]n
2.10. Crystallography
2.11. [Cu2(μ-OAc)4(1)]n
2.12. [{Cu4(μ3-OH)2(μ-OAc)2(μ3-OAc)2(AcO-κO)2(1)2}·2MeOH]n
2.13. [2{Cu2(μ-OAc)4(2)}·1.25MeOH]n
2.14. [Cu2(μ-OAc)4(3)]n
2.15. [{Cu2(μ-OAc)4(4)}·0.2CHCl3]n
3. Results and Discussion
3.1. Synthesis and Characterization of Ligands 3 and 4
3.2. Reactions of Copper(II) Acetate with Ligand 1
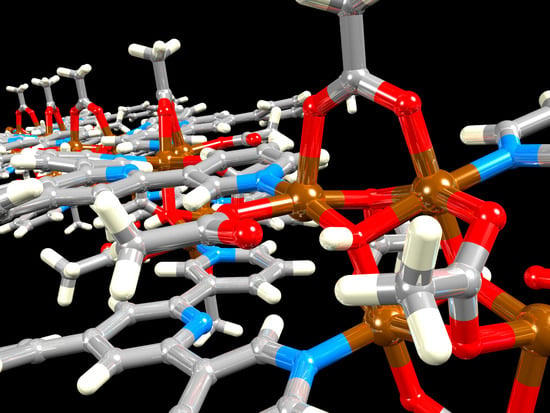
3.3. Crystal Structure of [Cu2(μ-OAc)4(1)]n
3.4. Reactions of Copper(II) Acetate with Ligands 2, 3 and 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [Green Version]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary building blocks as the turning point in the development of the reticular chemistry of MOFs. Sci. Adv. 2018, 4, eaat9180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghi, O.M. Reticular Chemistry in All Dimensions. ACS Cent. Sci. 2019, 5, 1295–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köberl, M.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. From molecules to materials: Molecular paddle-wheel synthons of macromolecules, cage compounds and metal–organic frameworks. Dalton Trans. 2011, 40, 6834–6859. [Google Scholar] [CrossRef] [PubMed]
- Vagin, S.I.; Ott, A.K.; Rieger, B. Paddle-wheel zinc carboxylate clusters as building units for metal-organic frameworks. Chem. Ing. Tech. 2007, 79, 767–780. [Google Scholar] [CrossRef]
- Handa, M.; Mikuriya, M.; Nukada, R.; Matsumoto, H.; Kasuga, K. Chain Compound of Molybdenum(II) Pivalate Bridged by 4,4′-Bipyridine. Bull. Chem. Soc. Jpn. 1994, 67, 3125–3127. [Google Scholar] [CrossRef]
- Yao, Q.; Sun, J.; Li, K.; Su, J.; Peskov, M.V.; Zou, X. A series of isostructural mesoporous metal–organic frameworks obtained by ion-exchange induced single-crystal to single-crystal transformation. Dalton Trans. 2012, 41, 3953–3955. [Google Scholar] [CrossRef]
- Chen, B.; Xiang, S.; Qian, G. Metal−Organic Frameworks with Functional Pores for Recognition of Small Molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal-Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Ligand and Metalloligand Design for Macrocycles, Multimetallic Arrays, Coordination Polymers and Assemblies; Reedijk, J., Ed.; Elsevier: Amsterdam, The Netherlands. [CrossRef]
- Constable, E.C.; Housecroft, C.E. Tetratopic bis(4,2′:6′,4″-terpyridine) and bis(3,2′:6′,3″-terpyridine) ligands as 4-connecting nodes in 2D-coordination networks and 3D-frameworks. J. Inorg. Organomet. Polym. Mater. 2018, 28, 414–427. [Google Scholar] [CrossRef] [Green Version]
- Housecroft, C.E.; Constable, E.C. Ditopic and tetratopic 4,2′:6′,4″-Terpyridines as Structural Motifs in 2D- and 3D-Coordination Assemblies. Chimia 2019, 73, 462–467. [Google Scholar] [CrossRef]
- Housecroft, C.E. 4,2′:6′,4″-Terpyridines: Diverging and Diverse Building Blocks in Coordination Polymers and Metallomacrocycles. Dalton Trans. 2014, 43, 6594–6604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Housecroft, C.E. Divergent 4,2′:6′,4″- and 3,2′:6′,3″-Terpyridines as Linkers in 2- and 3-Dimensional Architectures. CrystEngComm 2015, 17, 7461–7468. [Google Scholar] [CrossRef] [Green Version]
- Dorofeeva, V.N.; Mishura, A.M.; Lytvynenko, A.S.; Grabovaya, N.V.; Kiskin, M.A.; Kolotilov, S.V.; Eremenko, I.L.; Novotortsev, V.M. Structure and Electrochemical Properties of Copper(II) Coordination Polymers with Ligands Containing Naphthyl and Anthracyl Fragments. Theor. Exper. Chem. 2016, 52, 111–118. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Kopecky, P.; Neuburger, M.; Zampese, J.A.; Zhang, G. Coordination polymers with divergent 4′-tert-butyl-4,2′:6′,4″-terpyridine linkers: from aryl-aryl to ball-and-socket packing. CrystEngComm. 2012, 14, 446–452. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Vujovic, S.; Zampese, J.A.; Crochet, A.; Batten, S.R. Do perfluoroarene…arene and C–H...F interactions make a difference to 4,2′:6′,4″-terpyridine-based coordination polymers? CrystEngComm 2013, 15, 10068–10078. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Jia, Y.-X.; Chen, W.; Lo, W.-F.; Brathwaite, N.; Golen, J.A.; Rheingold, A.L. Diverse zinc(II) coordination assemblies built on divergent 4,2′:6′,4″-terpyridine derivatives: syntheses, structures and catalytic properties. RSC Adv. 2015, 5, 15870–15879. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Schönle, J.; Vujovic, S.; Zampese, J.A. Molecular recognition between 4′-(4-biphenylyl)-4,2′:6′,4″-terpyridine domains in the assembly of d9 and d10 metal ion-containing one-dimensional coordination polymers. Polyhedron 2013, 60, 120–129. [Google Scholar] [CrossRef]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Zampese, J.A.; Crochet, A. Greasy tails switch 1D-coordination [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4″-tpy)]n polymers to discrete [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4″-tpy)2] complexes. CrystEngComm 2014, 16, 9915–9929. [Google Scholar] [CrossRef] [Green Version]
- Constable, E.C.; Zhang, G.; Coronado, E.; Housecroft, C.E.; Neuburger, M. Not just size and shape: spherically symmetrical d5 and d10 metal ions give different coordination nets with 4,2′:6′,4″-terpyridines. CrystEngComm 2010, 12, 2139–2145. [Google Scholar] [CrossRef]
- Song, J.; Wang, B.-C.; Hu, H.-M.; Gou, L.; Wu, Q.-R.; Yang, X.-L.; Shangguan, Y.-Q.; Dong, F.-X.; Xue, G.-L. In situ hydrothermal syntheses, crystal structures and luminescent properties of two novel zinc(II) coordination polymers based on tetrapyridyl ligand. Inorg. Chim. Acta 2011, 366, 134–140. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.Z.; Yang, C.; Liu, E.; Golen, J.A.; Zhang, G. One-dimensional copper(II) coordination polymers built on 4′-substituted 4,2′:6′,4″- and 3,2′:6′,3″-terpyridines: Syntheses, structures and catalytic properties. Polyhedron 2016, 105, 115–122. [Google Scholar] [CrossRef]
- Tian, Y.; Xiao, L. CSD Communication, refcode CACXEM, 2015. [CrossRef]
- Xiao, L.; Tian, Y. CSD Communication, refcode VUKMOF, 2015. [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Vujovic, S.; Zampese, J.A.; Zhang, G. Cobalt(II) coordination polymers with 4′-substituted 4,2′:6′,4″- and 3,2′:6′,3″-terpyridines: engineering a switch from planar to undulating chains and sheet. CrystEngComm. 2012, 14, 3554–3563. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Wang, J.; Wu, W.-P.; Ma, A.; Liu, J.-Q.; Yadav, R.; Kumar, A. Fluorescent sensing of nitroaromatics by two coordination polymers having potential active sites. J. Luminescence 2017, 186, 40–47. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Coordination behaviour of 1-(4,2′:6′,4″-terpyridin-4′-yl)ferrocene and 1-(3,2′:6′,3″-terpyridin-4′-yl)ferrocene: predictable and unpredictable assembly algorithms. Aust. J. Chem. 2017, 70, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-T.; Zhang, J.-L.; Hu, H.-M.; Cheng, Y.; Xue, L.-L.; Wang, X.; Wang, B. Syntheses, structures and luminescent properties of Zn/Cd coordination polymers based on 4′-(2-carboxyphenyl)-3,2′:6′,3″- terpyridine. Polyhedron 2018, 151, 43–50. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.-J.; He, J.-E.; Chen, Y.-Y.; Zheng, S.-R.; Fan, J.; Zhang, W.-G. Construction of New Coordination Polymers from 4′-(2,4-disulfophenyl)-3,2′:6′3″-terpyridine: Polymorphism, pH-dependent syntheses, structures, and properties. J. Solid State Chem. 2016, 233, 444–454. [Google Scholar] [CrossRef]
- Yang, P.; Wang, M.-S.; Shen, J.-J.; Li, M.-X.; Wang, Z.-X.; Shao, M.; He, X. Seven novel coordination polymers constructed by rigid 4-(4-carboxyphenyl)-terpyridine ligands: Synthesis, structural diversity, luminescence and magnetic properties. Dalton Trans. 2014, 43, 1460–1470. [Google Scholar] [CrossRef]
- Yoshida, J.; Nishikiori, S.; Yuge, H. Bis(3-cyano-pentane-2,4-dionato) Co(II) as a linear building block for coordination polymers: combinations with two polypyridines. J. Coord. Chem. 2013, 66, 2191–2200. [Google Scholar] [CrossRef]
- Rocco, D.; Prescimone, A.; Klein, Y.M.; Gawryluk, D.J.; Constable, E.C.; Housecroft, C.E. Competition in coordination assemblies: 1D-coordination polymer or 2D-nets based on Co(NCS)2 and 4′-(4-methoxyphenyl)-3,2′:6′,3″-terpyridine. Polymers 2019, 11, 1224. [Google Scholar] [CrossRef] [Green Version]
- Rocco, D.; Housecroft, C.E.; Constable, E.C. Synthesis of Terpyridines: Simple Reactions – What Could Possibly Go Wrong? Molecules 2019, 24, 1799. [Google Scholar] [CrossRef] [Green Version]
- Software for the Integration of CCD Detector System Bruker Analytical X-ray Systems. Bruker axs: Madison, WI, USA, (after 2013).
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with ShelXL. Acta Cryst. 2015, C27, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Chapuis, G. Superflip—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A Computer Program for Topological Analysis of Discrete Electron Densities. J. Appl. Cryst. 2012, 45, 575–580. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- LeBail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mat. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Cryst. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. WinPLOTR: A Windows tool for powder diffraction patterns analysis Materials Science Forum. In Proceedings of the Seventh European Powder Diffraction Conference (EPDIC 7), Barcelona, Spain, 20–23 May 2000; pp. 118–123. [Google Scholar]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-hindered 4′-Aryl-2,2′:6′,2″- terpyridines. Synlett 2005, 1251–1254. [Google Scholar] [CrossRef]
- Alsalme, A.; Ghazzali, M.; Khan, R.A.; Al-Farhan, K.; Reedijk, J. A novel trinuclear μ3-hydroxido-bridged Cu(II) compound; a molecular cluster, stabilized by hydrogen bonding, bridging pyrazolates, terminal pyrazoles, water and nitrate anions. Polyhedron 2014, 72, 64–67. [Google Scholar] [CrossRef]
- Bette, S.; Kremer, R.K.; Eggert, G.; Tang, C.C.; Dinnebier, R.E. On verdigris, part I: synthesis, crystal structure solution and characterisation of the 1–2–0 phase (Cu3(CH3COO)2OH)4). Dalton Trans. 2017, 46, 14847–14858. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, J.R.; Walker, W.R. Infrared Spectra of Hydroxy-Bridged Copper(II) Compounds. Inorg. Chem. 1965, 4, 1382–1386. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Lah, N.; Koller, J.; Giester, G.; Segedin, P.; Leban, I. Copper(II) carboxylates with 4-aminopyridine: neutral mononuclear structures, isomerism of aceto compounds and a novel tetranuclear structure. New J. Chem. 2002, 26, 933–938. [Google Scholar] [CrossRef]
- Mezei, G.; Rivera-Carrillo, M.; Raptis, R.G. Effect of copper-substitution on the structure and nuclearity of Cu(II)-pyrazolates: from trinuclear to tetra-, hexa- and polynuclear complexes. Inorg. Chim. Acta 2004, 357, 3721–3732. [Google Scholar] [CrossRef]
- de Campos, N.R.; Ribeiro, M.A.; Oliveira, W.X.C.; Reis, D.O.; Stumpf, H.O.; Doriguetto, A.C.; Machado, F.C.; Pinheiro, C.B.; Lloret, F.; Julve, M.; et al. Magneto-structural versitility of copper(II)-3-phenylpropionate coordination polymers with N-donor coligands. Dalton Trans. 2016, 45, 172–189. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.-H.; Cheng, R.-M.; Song, Y.; Li, Y.-Z.; Yu, Z.; Chen, X.-T.; Xue, Z.-L.; You, X.-Z. Syntheses, Structures, and Magnetic Properties of Unusual Nonlinear Polynuclear Copper(II) Complexes Containing Derivatives of 1,2,4-Triazole and Pivalate Ligands. Inorg. Chem. 2005, 44, 8011–8022. [Google Scholar] [CrossRef]





















| Coordination Polymer | Cu–O/Å | Cu–N/Å |
|---|---|---|
| [Cu2(μ-OAc)4(1)]n | 1.964(8), 1.977(8), 1.987(9), 1.987(9), 1.943(8), 1.984(9), 1.953(9), 1.965(9) | 2.150(9), 2.156(8) |
| [2{Cu2(μ-OAc)4(2)}·1.25MeOH]n | 1.9659(16), 1.9764(16), 1.9728(15), 1.9777(16), 1.9786(15), 1.9803(15), 1.9753(15), 1.9544(15), 1.9805(16), 1.9671(15), 1.9971(15), 1.9580(15), 1.9745(16), 1.9808(15), 1.9716(16), 1.9839(15) | 2.1672(18), 2.1717(17), 2.1875(17), 2.1709(17) |
| [Cu2(μ-OAc)4(3)]n | 1.964(4), 1.992(4), 1.959(4), 1.988(4), 1.951(4), 1.950(4), 1.992(4), 1.997(4) | 2.205(4), 2.161(4) |
| [{Cu2(μ-OAc)4(4)}·0.2CHCl3]n | 1.963(2), 1.967(2), 1.976(2), 1.976(2), 1.976(2), 1.974(2), 1.982(2), 1.972(2) | 2.163(3), 2.149(3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, D.; Manfroni, G.; Prescimone, A.; Klein, Y.M.; Gawryluk, D.J.; Constable, E.C.; Housecroft, C.E. Single and Double-Stranded 1D-Coordination Polymers with 4′-(4-Alkyloxyphenyl)-3,2′:6′,3″-terpyridines and {Cu2(μ-OAc)4} or {Cu4(μ3-OH)2(μ-OAc)2(μ3-OAc)2(AcO-κO)2} Motifs. Polymers 2020, 12, 318. https://doi.org/10.3390/polym12020318
Rocco D, Manfroni G, Prescimone A, Klein YM, Gawryluk DJ, Constable EC, Housecroft CE. Single and Double-Stranded 1D-Coordination Polymers with 4′-(4-Alkyloxyphenyl)-3,2′:6′,3″-terpyridines and {Cu2(μ-OAc)4} or {Cu4(μ3-OH)2(μ-OAc)2(μ3-OAc)2(AcO-κO)2} Motifs. Polymers. 2020; 12(2):318. https://doi.org/10.3390/polym12020318
Chicago/Turabian StyleRocco, Dalila, Giacomo Manfroni, Alessandro Prescimone, Y. Maximilian Klein, Dariusz J. Gawryluk, Edwin C. Constable, and Catherine E. Housecroft. 2020. "Single and Double-Stranded 1D-Coordination Polymers with 4′-(4-Alkyloxyphenyl)-3,2′:6′,3″-terpyridines and {Cu2(μ-OAc)4} or {Cu4(μ3-OH)2(μ-OAc)2(μ3-OAc)2(AcO-κO)2} Motifs" Polymers 12, no. 2: 318. https://doi.org/10.3390/polym12020318
APA StyleRocco, D., Manfroni, G., Prescimone, A., Klein, Y. M., Gawryluk, D. J., Constable, E. C., & Housecroft, C. E. (2020). Single and Double-Stranded 1D-Coordination Polymers with 4′-(4-Alkyloxyphenyl)-3,2′:6′,3″-terpyridines and {Cu2(μ-OAc)4} or {Cu4(μ3-OH)2(μ-OAc)2(μ3-OAc)2(AcO-κO)2} Motifs. Polymers, 12(2), 318. https://doi.org/10.3390/polym12020318