Application Progress of Polyaniline, Polypyrrole and Polythiophene in Lithium-Sulfur Batteries
Abstract
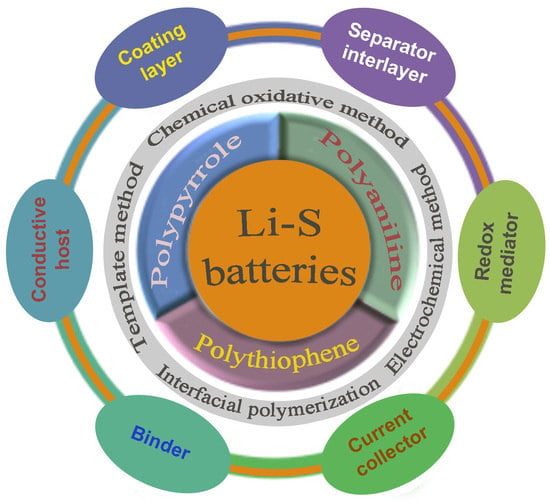
:1. Introduction
2. Application of PANI in Li-S Batteries
2.1. Coating Layer
2.2. Conductive Host
2.3. Covalent Bond Compounds
2.4. Separator Modifier/Interlayer
2.5. Redox Mediator
3. Application of PPy in Li-S Batteries
3.1. Coating Layer
3.2. Conductive Host
3.3. Separator Modifier/Functional Interlayer
3.4. Binder
3.5. Current Collector
4. Application of PTh and Its Derivatives
4.1. Coating Layer
4.2. Sulfur-Containing Copolymer
4.3. Functional Interlayer
4.4. Binder
5. Summary and Perspectives
5.1. Summary
5.2. Perspectives
- (1)
- Developing controllable synthetic techniques for conducting polymers. The species and microstructures of conducting polymers decide their performance and application. Generally speaking, the performance of Li-S batteries depends on the thickness of the coating layer, the types and microstructure of conducting polymers. Therefore, suitable synthetic methods and corresponding doping treatments are always a research topic for conducting polymers.
- (2)
- Designing hybrid wrapping layers based on chemical anchoring mechanisms. Compared to single layers of conducting polymers, double-layer coatings on sulfur cathodes achieve the synergistic effect of different coatings, and greatly improve the cell performance. For example, PPy and MnO2 hybrid layers on a sulfur cathode exhibited stable cycling for 200 cycles. Therefore, the combination of conducting polymers with metal oxide layers will become a promising research direction.
- (3)
- Synthesizing high-performance sulfur-containing compounds. Because covalent bonds can be formed between sulfur and conducting polymers, while the covalent bonds also play a crucial role in anchoring sulfur, therefore, various sulfur-containing compounds have been synthesized for Li-S batteries, such as, SPANI, S3BT, and S-PMAT copolymers. Developing sulfur-containing compounds will become a hot topic in the commercialization of conducting polymers and Li-S batteries.
- (4)
- Exploring novel chemical anchoring or electrocatalysis mechanisms of conducting polymers. The performance enhancement of Li-S batteries is mostly attributed to the physical confinement and chemical anchoring. However, the specific interaction mechanism of sulfur confinement is very complicated. For example, the quinonoid imine groups (-NH+=/-N=) in the conductive copolymers have an electrocatalytic effect on the redox reaction of sulfur species. Therefore, it is an essential task to explore the relationship between the functional groups of conducting polymers and reaction mechanism of Li-S batteries, which will provide a theoretical guidance to develop novel conductive copolymers.
Author Contributions
Funding
Conflicts of Interest
References
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building Better Batteries in the Solid State: A Review. Materials 2019, 12, 3892. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ma, L.; Ren, J.; Zhang, M.; Luo, X.; Li, B.; Song, Z.; Zhou, X. Wheat Straw-Derived N-, O-, and S-Tri-doped Porous Carbon with Ultrahigh Specific Surface Area for Lithium-Sulfur Batteries. Materials 2018, 11, 989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.; Wang, R.; Liu, Y.; Fu, J.; Liang, J.; Dou, S. Recent advances in chemical adsorption and catalytic conversion materials for Li–S batteries. J. Energy Chem. 2020, 42, 144–168. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Long, J.; Du, S.; Sun, B.; Zhu, S.; Li, J. Three-Dimensionally Porous Li-Ion and Li-S Battery Cathodes: A Mini Review for Preparation Methods and Energy-Storage Performance. Nanomaterials 2019, 9, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Manthiram, A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy Storage Mater. 2019, 20, 55–70. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, S.; Wei, Y.; Bian, H.; Wang, R.; Min, Y. Lignin Nanoparticle-Coated Celgard Separator for High-Performance Lithium–Sulfur Batteries. Polymers 2019, 11, 1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured conducting polymers for advanced energy storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, H.; Shah, A.-U.-H.A.; Bilal, S. Achieving Ultrahigh Cycling Stability and Extended Potential Window for Supercapacitors through Asymmetric Combination of Conducting polymer Nanocomposite and Activated Carbon. Polymers 2019, 11, 1678. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Fu, J.; Liu, Y.; Li, S.; Wang, X.; Dong, W.; Yang, S. Recent Progress on Graphene/Polyaniline Composites for High-performance Supercapacitors. Materials 2019, 12, 1451. [Google Scholar] [CrossRef] [Green Version]
- Souza, V.H.R.; Oliveira, M.M.; Zarbin, A.J.G. Bottom-up synthesis of graphene/polyaniline nanocomposites for flexible and transparent energy storage devices. J. Power Sources 2017, 348, 87–93. [Google Scholar] [CrossRef]
- Yu, J.; Xie, F.; Wu, Z.; Huang, T.; Wu, J.; Yan, D.; Huang, C.; Li, L. Flexible metallic fabric supercapacitor based on graphene/polyaniline composites. Electrochim. Acta 2018, 259, 968–974. [Google Scholar] [CrossRef]
- Li, H.; Song, J.; Wang, L.; Feng, X.; Liu, R.; Zeng, W.; Huang, Z.; Ma, Y.; Wang, L. Flexible all-solid-state supercapacitors based on polyaniline orderly nanotubes array. Nanoscale 2017, 9, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hou, C.; Zhang, H.; Qiao, M.; Chen, Y.; Zhang, H.; Zhang, Q.; Guo, Z. Morphology-dependent electrochemical supercapacitors in multi-dimensional polyaniline nanostructures. J. Mater. Chem. A 2017, 5, 14041–14052. [Google Scholar] [CrossRef]
- Wang, R.; Han, M.; Zhao, Q.; Ren, Z.; Guo, X.; Xu, C.; Hu, N.; Lu, L. Hydrothermal synthesis of nanostructured graphene/polyaniline composites as high-capacitance electrode materials for supercapacitors. Sci. Rep. 2017, 7, 44562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Kaner, R.B. A General Chemical Route to Polyaniline Nanofibers. J. Am. Chem. Soc. 2004, 126, 851–855. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, S.; Liu, W.; Cheng, H.; Chen, S.; Liu, X.; Liu, J.; Tai, Q.; Hu, C. Facile Fabrication of Urchin-like Polyaniline Microspheres for Electrochemical Energy Storage. Electrochim. Acta 2017, 254, 25–35. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Q.; Mi, H. Flexible and cross-linked polyaniline nets as promising supercapacitor electrodes. Mater. Lett. 2016, 163, 115–117. [Google Scholar] [CrossRef]
- Duan, L.; Lu, J.; Liu, W.; Huang, P.; Wang, W.; Liu, Z. Fabrication of conducting polymer-coated sulfur composite cathode materials based on layer-by-layer assembly for rechargeable lithium–sulfur batteries. Colloid Surf. A 2012, 414, 98–103. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Lu, Y.; Zhu, Y.; Qian, Y.; Wang, D.; Zhou, J.; Lin, N.; Qian, Y. A graphene oxide-wrapped bipyramidal sulfur@polyaniline core–shell structure as a cathode for Li–S batteries with enhanced electrochemical performance. J. Mater. Chem. A 2016, 4, 6404–6410. [Google Scholar] [CrossRef]
- An, Y.; Wei, P.; Fan, M.; Chen, D.; Chen, H.; Ju, Q.; Tian, G.; Shu, K. Dual-shell hollow polyaniline/sulfur-core/polyaniline composites improving the capacity and cycle performance of lithium–sulfur batteries. Appl. Surf. Sci. 2016, 375, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, S.; Cheng, H.; Dai, Y.; Yu, J.; Wu, J. Synthesis of a ternary polyaniline@acetylene black-sulfur material by continuous two-step liquid phase for lithium sulfur batteries. Electrochim. Acta 2015, 158, 143–151. [Google Scholar] [CrossRef]
- Wang, M.; Wang, W.; Wang, A.; Yuan, K.; Miao, L.; Zhang, X.; Huang, Y.; Yu, Z.; Qiu, J. A multi-core–shell structured composite cathode material with a conducting polymer network for Li–S batteries. Chem. Commun. 2013, 49, 10263–10265. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wen, Z.; Ma, G.; Lu, Y.; Rui, K. Mesoporous carbon/sulfur composite with polyaniline coating for lithium sulfur batteries. Solid State Ion. 2014, 262, 170–173. [Google Scholar] [CrossRef]
- Ding, K.; Bu, Y.; Liu, Q.; Li, T.; Meng, K.; Wang, Y. Ternary-layered nitrogen-doped graphene/sulfur/polyaniline nanoarchitecture for the high-performance of lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 8022–8027. [Google Scholar] [CrossRef]
- Ding, Z.W.; Zhao, D.L.; Yao, R.R.; Li, C.; Cheng, X.W.; Hu, T. Polyaniline@spherical ordered mesoporous carbon/sulfur nanocomposites for high-performance lithium-sulfur batteries. Int. J. Hydrogen Energy 2018, 43, 10502–10510. [Google Scholar] [CrossRef]
- Hu, H.; Cheng, H.; Liu, Z.; Li, G.; Zhu, Q.; Yu, Y. In Situ Polymerized PAN-Assisted S/C Nanosphere with Enhanced High-Power Performance as Cathode for Lithium/Sulfur Batteries. Nano Lett. 2015, 15, 5116–5123. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Chen, L.; Lu, Y.; Su, Y.; Li, J.; Bao, L.; Yao, J.; Zhou, Y.; Chen, R. Electron bridging structure glued yolk-shell hierarchical porous carbon/sulfur composite for high performance Li-S batteries. Electrochim. Acta 2018, 292, 199–207. [Google Scholar] [CrossRef]
- Li, X.; Rao, M.; Chen, D.; Lin, H.; Liu, Y.; Liao, Y.; Xing, L.; Li, W. Sulfur supported by carbon nanotubes and coated with polyaniline: Preparation and performance as cathode of lithium-sulfur cell. Electrochim. Acta 2015, 166, 93–99. [Google Scholar] [CrossRef]
- Kim, J.H.; Fu, K.; Choi, J.; Kil, K.; Kim, J.; Han, X.; Hu, L.; Paik, U. Encapsulation of S/SWNT with PANI Web for Enhanced Rate and Cycle Performance in Lithium Sulfur Batteries. Sci. Rep. 2015, 5, 8946. [Google Scholar] [CrossRef]
- Zhu, P.; Zhu, J.; Yan, C.; Dirican, M.; Zang, J.; Jia, H.; Li, Y.; Kiyak, Y.; Tan, H.; Zhang, X. In Situ Polymerization of Nanostructured Conducting polymer on 3D Sulfur/Carbon Nanofiber Composite Network as Cathode for High-Performance Lithium–Sulfur Batteries. Adv. Mater. Interfaces 2018, 5, 1701598. [Google Scholar] [CrossRef]
- Moon, S.; Jung, Y.H.; Kim, D.K. Enhanced electrochemical performance of a crosslinked polyaniline-coated graphene oxide-sulfur composite for rechargeable lithium–sulfur batteries. J. Power Sources 2015, 294, 386–392. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, W.; Li, G.; Hou, Y.; Zhou, L.; Li, H.; Liu, M.; Ye, F.; Yang, X.; Zhang, Y. Polyaniline-modified cetyltrimethylammonium bromide-graphene oxide-sulfur nanocomposites with enhanced performance for lithium-sulfur batteries. Nano Res. 2014, 7, 1355–1363. [Google Scholar] [CrossRef]
- Lu, Q.; Gao, H.; Yao, Y.; Liu, N.; Wang, X.; Wang, F. One-step synthesis of an urchin-like sulfur/polyaniline nano-composite as a promising cathode material for high-capacity rechargeable lithium–sulfur batteries. RSC Adv. 2015, 5, 92918–92922. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Q.; Liu, N.; Wang, X.; Wang, F. Facile preparation of an ultrathin sulfur-wrapped polyaniline nanofiber composite with a core–shell structure as a high performance cathode material for lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 7215–7218. [Google Scholar] [CrossRef]
- Zhao, X.; Ahn, H.J.; Kim, K.W.; Cho, K.K.; Ahn, J.H. Polyaniline-Coated Mesoporous Carbon/Sulfur Composites for Advanced Lithium Sulfur Batteries. J. Phys. Chem. C 2015, 119, 7996–8003. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, J.K.; Ahn, H.J.; Cho, K.K.; Ahn, J.H. A ternary sulfur/polyaniline/carbon composite as cathode material for lithium sulfur batteries. Electrochim. Acta 2013, 109, 145–152. [Google Scholar] [CrossRef]
- Wang, J.; Yue, K.; Zhu, X.; Wang, K.L.; Duan, L. C–S@PANI composite with a polymer spherical network structure for high performance lithium–sulfur batteries. Phys. Chem. Chem. Phys. 2016, 18, 261–266. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Lu, Y.; Wu, X.; Wu, M.; Chen, C. Hollow polyaniline sphere@ sulfur composites for prolonged cycling stability of lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 10350–10354. [Google Scholar] [CrossRef]
- Wei, P.; Fan, M.Q.; Chen, H.C.; Yang, X.R.; Wu, H.M.; Chen, J.; Li, T.; Zeng, L.W.; Li, C.M.; Ju, Q.J.; et al. Enhanced cycle performance of hollow polyaniline sphere/sulfur composite in comparison with pure sulfur for lithium–sulfur batteries. Renew. Energy 2016, 86, 148–153. [Google Scholar] [CrossRef]
- Deng, H.; Yao, L.; Huang, Q.A.; Su, Q.; Zhang, J.; Zhang, F.; Du, G. Facile assembly of a S@carbon nanotubes/polyaniline/graphene composite for lithium–sulfur batteries. RSC Adv. 2017, 7, 9819–9825. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, Q.; Lai, Y.; Li, J. Confine Sulfur in Polyaniline-Decorated Hollow Carbon Nanofiber Hybrid Nanostructure for Lithium–Sulfur Batteries. J. Phys. Chem. C 2014, 118, 13369–13376. [Google Scholar] [CrossRef]
- Li, L.; Ruan, G.; Peng, Z.; Yang, Y.; Fei, H.; Raji, A.R.O.; Samuel, E.L.G.; Tour, J.M. Enhanced Cycling Stability of Lithium Sulfur Batteries Using Sulfur–Polyaniline–Graphene Nanoribbon Composite Cathodes. ACS Appl. Mater. Interfaces 2014, 6, 15033–15039. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Liu, X.; Guo, J.; Pan, L.; Wang, H.; Su, Q.; Du, G. Nanosulfur/polyaniline/graphene composites for high-performance lithium–sulfur batteries: One pot in-situ synthesis. Mater. Lett. 2014, 133, 193–196. [Google Scholar] [CrossRef]
- Yan, J.; Li, B.; Liu, X. Nano-porous sulfur–polyaniline electrodes for lithium–sulfurbatteries. Nano Energy 2015, 18, 245–252. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yan, X.; Qu, Y.; Lai, Y.; Li, J. Interface polymerization synthesis of conducting polymer/graphite oxide@sulfur composites for high-rate lithium-sulfur batteries. Electrochim. Acta 2015, 155, 54–60. [Google Scholar] [CrossRef]
- Tsao, C.H.; Hsu, C.H.; Zhou, J.D.; Chin, C.W.; Kuo, P.L.; Chang, C.H. Vulcanized polymeric cathode material featuring a polyaniline skeleton for high-rate rechargeability and long-cycle stability lithium-sulfur batteries. Electrochim. Acta 2018, 276, 111–117. [Google Scholar] [CrossRef]
- Chang, C.H.; Chung, S.H.; Manthiram, A. Ultra-lightweight PANiNF/MWCNT-functionalized separators with synergistic suppression of polysulfide migration for Li–S batteries with pure sulfur cathodes. J. Mater. Chem. A 2015, 3, 18829–18834. [Google Scholar] [CrossRef]
- Moon, S.; Yoo, J.K.; Jung, Y.H.; Kim, J.H.; Jung, Y.S.; Kim, D.K. Effective Suppression of Polysulfide Dissolution by Uniformly Transfer-Printed Conducting Polymer on Sulfur Cathode for Li-S Batteries. J. Electrochem. Soc. 2017, 164, A6417–A6421. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Peng, H.J.; Hou, T.Z.; Zhai, P.Y.; Li, B.Q.; Tang, C.; Zhu, W.; Huang, J.Q.; Zhang, Q. A Quinonoid-Imine-Enriched Nanostructured Polymer Mediator for Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1606802. [Google Scholar] [CrossRef]
- Oh, E.J.; Jang, K.S.; MacDiarmid, A.G. High molecular weight soluble polypyrrole. Synth. Met. 2001, 125, 267–272. [Google Scholar] [CrossRef]
- Nishio, K.; Fujimoto, M.; Ando, O.; Ono, H.; Murayama, T. Characteristics of polypyrrole chemically synthesized by various oxidizing reagents. J. Appl. Electrochem. 1996, 26, 425–429. [Google Scholar] [CrossRef]
- Wu, T.M.; Chang, H.L.; Lin, Y.W. Synthesis and characterization of conductive polypyrrole with improved conductivity and processability. Polym. Int. 2009, 58, 1065–1070. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yoneyama, H.; Tamura, H. Polyaniline film-coated electrodes as electrochromic display devices. J. Electroanal. Chem. Interfacial Electrochem. 1984, 161, 419–423. [Google Scholar] [CrossRef]
- Nakayama, M.; Yano, J.; Nakaoka, K.; Ogura, K. Electrodeposition of composite films consisting of polypyrrole and mesoporous silica. Synth. Met. 2002, 128, 57–62. [Google Scholar] [CrossRef]
- Geetha, S.; Trivedi, D.C. Studies on polypyrrole film in room temperature melt. Mater. Chem. Phys. 2004, 88, 388–397. [Google Scholar] [CrossRef]
- Vito, S.D.; Martin, C.R. Toward Colloidal Dispersions of Template-synthesized Polypyrrole Nanotubules. Chem. Mater. 1998, 10, 1738–1741. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, Y.; Yang, L. Adjusting the inner-structure of polypyrrole nanoparticles through microemulsion polymerization. Mater. Chem. Phys. 2006, 98, 304–308. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Gosselink, D.; Li, Z.; Ghaznavi, M.; Chen, P. One-pot approach to synthesize PPy@ S core–shell nanocomposite cathode for Li/S batteries. J. Nanopart. Res. 2013, 15, 2007. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, H. Facile synthesis and performance of polypyrrole-coated sulfur nanocomposite as cathode materials for lithium/sulfur batteries. J. Energy Chem. 2014, 23, 657–661. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, H.; Cheng, H.; Hu, C.; Fang, W.; Fang, J.; Xu, J.; Chen, Z. Facile large-scale synthesis of core–shell structured sulfur@polypyrrole composite and its application in lithium–sulfur batteries with high energy density. Appl. Energy 2016, 175, 522–528. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, F.; Yang, J. Core@ shell sulfur@polypyrrole nanoparticles sandwiched in graphene sheets as cathode for lithium–sulfur batteries. J. Energy Chem. 2015, 24, 448–455. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Ding, Y.; Zhang, W.; Yu, G. In Situ Reactive Synthesis of Polypyrrole-MnO2 Coaxial Nanotubes as Sulfur Hosts for High-Performance Lithium–Sulfur Battery. Nano Lett. 2016, 16, 7276–7281. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, M.; Kaiser, M.R.; Gao, X.; Konstantinov, K.; Tandiono, R.; Wang, Z.; Liu, H.K.; Dou, S.X.; Wang, J. Split-half-tubular polypyrrole@ sulfur@ polypyrrole composite with a novel three-layer-3D structure as cathode for lithium/sulfur batteries. Nano Energy 2015, 11, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Jin, B.; Li, H.; Dong, C.; Zhang, B.; Xu, J.; Jiang, Q. Synergistic effect of tubular amorphous carbon and polypyrrole on polysulfides in Li-S batteries. J. Electroanal. Chem. 2017, 806, 41–49. [Google Scholar] [CrossRef]
- Wang, J.; Lu, L.; Shi, D.; Tandiono, R.; Wang, Z.; Konstantinov, K.; Liu, H. A Conductive Polypyrrole-Coated, Sulfur–Carbon Nanotube Composite for Use in Lithium–Sulfur Batteries. ChemPlusChem 2013, 78, 318–324. [Google Scholar] [CrossRef]
- Wang, C.; Wan, W.; Chen, J.T.; Zhou, H.H.; Zhang, X.X.; Yuan, L.X.; Huang, Y.H. Dual core–shell structured sulfur cathode composite synthesized by a one-pot route for lithium sulfur batteries. J. Mater. Chem. A 2013, 1, 1716–1723. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.; Li, L.; Zhao, T.; Liu, Z.; Chen, R. Polyethylene-Glycol-Doped Polypyrrole Increases the Rate Performance of the Cathode in Lithium–Sulfur Batteries. ChemSusChem 2013, 6, 1438–1444. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, S.; Wang, Z.; Liu, Y.; Zhao, Z.; Qiu, J. Sulfur-infiltrated graphene-backboned mesoporous carbon nanosheets with a conducting polymer coating for long-life lithium–sulfur batteries. Nanoscale 2015, 7, 7569–7573. [Google Scholar] [CrossRef]
- Lang, J.W.; Yan, X.B.; Yuan, X.Y.; Yang, J.; Xue, Q.J. Study on the electrochemical properties of cubic ordered mesoporous carbon for supercapacitors. J. Power Sources 2011, 196, 10472–10478. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Wang, Q.; Shen, C.; Peng, P.; Jin, J.; Wu, X. Enhanced performance of lithium sulfur battery with self-assembly polypyrrole nanotube film as the functional interlayer. J. Power Sources 2015, 273, 511–516. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, S.; Jiang, T.; Zhang, L.; Yu, J. Nano-wire networks of sulfur–polypyrrole composite cathode materials for rechargeable lithium batteries. Electrochem. Commun. 2008, 10, 1819–1822. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, S.; Zhang, L.; Sun, M.; Wang, W. Preparation and enhanced electrochemical properties of nano-sulfur/poly(pyrrole-co-aniline) cathode material for lithium/sulfur batteries. Electrochim. Acta 2010, 55, 4632–4636. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Lu, Y.; Wu, X.; Liu, C.; Chen, C. Enhancement of long stability of Li–S battery by thin wall hollow spherical structured polypyrrole based sulfur cathode. RSC Adv. 2014, 4, 21612–21618. [Google Scholar] [CrossRef]
- Liang, X.; Wen, Z.; Liu, Y.; Zhang, H.; Jin, J.; Wu, M.; Wu, X. A composite of sulfur and polypyrrole–multi walled carbon combinatorial nanotube as cathode for Li/S battery. J. Power Sources 2012, 206, 409–413. [Google Scholar] [CrossRef]
- Qian, W.; Gao, Q.; Zhang, H.; Tian, W.; Li, Z.; Tan, Y. Crosslinked Polypyrrole Grafted Reduced Graphene Oxide-Sulfur Nanocomposite Cathode for High Performance Li-S Battery. Electrochim. Acta 2017, 235, 32–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Gosselink, D.; Soboleski, H.G.; Chen, P. A novel nano-sulfur/polypyrrole/graphene nanocomposite cathode with a dual-layered structure for lithium rechargeable batteries. J. Power Sources 2013, 241, 517–521. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, K.; Yuan, K.; Lu, W.; Hu, S.; Wei, W.; Bai, M.; Shen, C. Enabling effective polysulfide trapping and high sulfur loading via a pyrrole modified graphene foam host for advanced lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 7309–7315. [Google Scholar] [CrossRef]
- Xin, P.; Jin, B.; Li, H.; Lang, X.; Yang, C.; Gao, W.; Zhu, Y.; Zhang, W.; Dou, S.; Jiang, Q. Facile Synthesis of Sulfur–Polypyrrole as Cathodes for Lithium–Sulfur Batteries. ChemElectroChem 2017, 4, 115–121. [Google Scholar] [CrossRef]
- Yin, F.; Liu, X.; Zhang, Y.; Zhao, Y.; Menbayeva, A.; Bakenov, Z.; Wang, X. Well-dispersed sulfur anchored on interconnected polypyrrole nanofiber network as high performance cathode for lithium-sulfur batteries. Solid State Sci. 2017, 66, 44–49. [Google Scholar] [CrossRef]
- Ma, G.; Huang, F.; Wen, Z.; Wang, Q.; Hong, X.; Jin, J.; Wu, X. Enhanced performance of lithium sulfur batteries with conducting polymer modified separators. J. Mater. Chem. A 2016, 4, 16968–16974. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Lu, Y.; Rui, K.; Wu, X.; Wu, M.; Zhang, J. Enhanced performance of lithium sulfur battery with polypyrrole warped mesoporous carbon/sulfur composite. J. Power Sources 2014, 254, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Manthiram, A. Enhanced Cyclability of Lithium–Sulfur Batteries by a Polymer Acid-Doped Polypyrrole Mixed Ionic–Electronic Conductor. Chem. Mater. 2012, 24, 3081–3087. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Wu, M.; Wu, X.; Zhang, J. Enhanced cycle performance of Li–S battery with a polypyrrole functional interlayer. J. Power Sources 2014, 267, 542–546. [Google Scholar] [CrossRef]
- Milroy, C.; Manthiram, A. An Elastic, Conductive, Electroactive Nanocomposite Binder for Flexible Sulfur Cathodes in Lithium–Sulfur Batteries. Adv. Mater. 2016, 28, 9744–9751. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Kaiser, M.R.; Ma, J.; Guo, Z.; Liu, H.; Wang, J. Free-standing sulfur-polypyrrole cathode in conjunction with polypyrrole-coated separator for flexible Li-S batteries. Energy Storage Mater. 2018, 13, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Gök, A.; Omastová, M.; Yavuz, A.G. Synthesis and characterization of polythiophenes prepared in the presence of surfactants. Synth. Met. 2007, 157, 23–29. [Google Scholar] [CrossRef]
- Pringle, J.M.; Forsyth, M.; MacFarlane, D.R.; Wagner, K.; Hall, S.B.; Officer, D.L. The influence of the monomer and the ionic liquid on the electrochemical preparation of polythiophene. Polymer 2005, 46, 2047–2058. [Google Scholar] [CrossRef]
- Lee, S.L.; Chang, C.J. Recent Developments about Conductive Polymer Based Composite Photocatalysts. Polymers 2019, 11, 206. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Dong, W.; Ge, J.; Wang, C.; Wu, X.; Lu, W.; Chen, L. Ultrafine Sulfur Nanoparticles in Conducting Polymer Shell as Cathode Materials for High Performance Lithium/Sulfur Batteries. Sci. Rep. 2013, 3, 1910. [Google Scholar] [CrossRef]
- Lee, J.; Choi, W. Surface Modification of Sulfur Cathodes with PEDOT:PSS Conducting Polymer in Lithium-Sulfur Batteries. J. Electrochem. Soc. 2015, 162, A935–A939. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, Y.; Li, Y.; Huo, Y.; Yu, Y.; Wang, C.; Jin, J.; Chen, L.; Hasan, T.; Wang, B.; et al. Manganese dioxide nanosheet functionalized sulfur@ PEDOT core–shell nanospheres for advanced lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 9403–9412. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, G.; Cha, J.J.; Wu, H.; Vosgueritchian, M.; Yao, Y.; Bao, Z.; Cui, Y. Improving the Performance of Lithium–Sulfur Batteries by Conducting polymer Coating. ACS Nano 2011, 5, 9187–9193. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Bu, F.; Yang, G.; Zhang, Y.; Xu, Y. Integration of Graphene, Nano Sulfur, and Conducting Polymer into Compact, Flexible Lithium–Sulfur Battery Cathodes with Ultrahigh Volumetric Capacity and Superior Cycling Stability for Foldable Devices. Adv. Mater. 2017, 29, 1703324. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.W.; Li, H.J.; Zou, J.Z.; Zeng, S.Z.; Li, Q.D.; Xu, G.Z.; Sheng, H.C.; Wang, B.B.; Si, Y.H.; Yu, L.; et al. Conducting polymer-coated MIL-101/S composite with scale-like shell structure for improving Li–S batteries. RSC Adv. 2018, 8, 4786–4793. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Cortie, M.; Fan, H.; Wang, G. Prussian Blue Nanocubes with an Open Framework Structure Coated with PEDOT as High-Capacity Cathodes for Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1700587. [Google Scholar] [CrossRef]
- Jeong, T.G.; Lee, Y.S.; Cho, B.W.; Kim, Y.T.; Jung, H.G.; Chung, K.Y. Improved performance of dual-conducting polymer-coated sulfur composite with high sulfur utilization for lithium-sulfur batteries. J. Alloys Compd. 2018, 742, 868–876. [Google Scholar] [CrossRef]
- Reddy, B.N.; Deepa, M.; Joshi, A.G.; Srivastava, A.K. Poly (3,4-Ethylenedioxypyrrole) Enwrapped by Reduced Graphene Oxide: How Conduction Behavior at Nanolevel Leads to Increased Electrochemical Activity. J. Phys. Chem. C 2011, 115, 18354–18365. [Google Scholar] [CrossRef]
- Mukkabla, R.; Meduri, P.; Deepa, M.; Shivaprasad, S.M.; Ghosal, P. Sulfur enriched carbon nanotubols with a Poly(3,4-ethylenedioxypyrrole) coating as cathodes for long-lasting Li-S batteries. J. Power Sources 2017, 342, 202–213. [Google Scholar] [CrossRef]
- Yu, B.C.; Jung, J.W.; Park, K.; Goodenough, J.B. A new approach for recycling waste rubber products in Li–S batteries. Energy Environ. Sci. 2017, 10, 86–90. [Google Scholar] [CrossRef]
- Zeng, S.; Li, L.; Zhao, D.; Liu, J.; Niu, W.; Wang, N.; Chen, S. Polymer-Capped Sulfur Copolymers as Lithium–Sulfur Battery Cathode: Enhanced Performance by Combined Contributions of Physical and Chemical Confinements. J. Phys. Chem. C 2017, 121, 2495–2503. [Google Scholar] [CrossRef]
- Zeng, S.; Li, L.; Xie, L.; Zhao, D.; Wang, N.; Chen, S. Conducting Polymers Crosslinked with Sulfur as Cathode Materials for High-Rate, Ultralong-Life Lithium–Sulfur Batteries. ChemSusChem 2017, 10, 3378–3386. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xu, G.; Ding, B.; Chang, Z.; Wang, Y.; Dou, H.; Zhang, X. Highly Conductive and Lightweight Composite Film as Polysulfide Reservoir for High-Performance Lithium–Sulfur Batteries. ChemElectroChem 2017, 4, 362–368. [Google Scholar] [CrossRef]
- Su, Y.S.; Manthiram, A. A new approach to improve cycle performance of rechargeable lithium–sulfur batteries by inserting a free-standing MWCNT interlayer. Chem. Commun. 2012, 48, 8817–8819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yuan, L.; Li, Z.; Qi, Y.; Wu, C.; Liu, J.; Huang, Y. Improving the electrochemical performance of a lithium–sulfur battery with a conducting polymer-coated sulfur cathode. RSC Adv. 2015, 5, 44160–44164. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Battaglia, V.; Liu, G. Improving the performance of lithium–sulfur batteries using conducting polymer and micrometric sulfur powder. J. Mater. Res. 2014, 29, 1027–1033. [Google Scholar] [CrossRef]









| Composite | Sulfur Content | Capacity (mAh g−1/rate) | Cycling Performance (mAh g−1/cycles/rate) | Ref. |
|---|---|---|---|---|
| S@PANI/GO | 54.3 wt.% | 1524/0.05 C | 875/100/0.2 C 641/300/1 C | [19] |
| hPANI/S/PANI | N/A | N/A | 572.2/214/0.1 C | [20] |
| PANI@S-C | N/A | 1257/0.16 mA cm−2 | 600/100/0.16 mA cm−2 | [21] |
| C-PANI–S@PANI | 87 wt.% 6 mg cm−2 | 1011/0.2 C | 835/100/0.2 C | [22] |
| CMK3/S-PANI | 48 wt.% | 1103/1 C | 649/100/1 C | [23] |
| PANI@S-OMC/S | N/A | 1626/0.1 C | 1338/100/0.1 C | [25] |
| PANI-assisted S/C nanosphere (PSCs-73) | 73 wt.% | N/A | 345/2500/5 C | [26] |
| HPC@S-PANI | N/A | 1372/0.2 A g−1 | 494.5/500/2A g−1 | [27] |
| MWCNTs-S@PANI | N/A | 970.8/0.2 C | 545.5/205/0.2 C | [28] |
| PANI-S/SWNT | 85 wt.% | 1415/0.2 C | 1011/100/0.2 C | [29] |
| 3D CNF/S/PANI | N/A | 1074/0.2 C | 935/300/0.2 C 552/300/1 C | [30] |
| GO-S@PANI | 75 wt.% | 1246/0.5 C | 80.43%/500/1 C | [31] |
| CTAB-GO-S | 0.8 mg cm−2 | 970/0.2 C 820/0.5 C 770/1 C | 715/300/0.2 C 670/500/0.5 C 570/500/1 C | [32] |
| NGNS-S-PANI | N/A | 1227.3/0.5 C | 693/100/0.5 C | [24] |
| S/PANI | 55 wt.% | 1095/0.1 C | 832/100/0.2 C 609/100/1 C | [33] |
| S-PANI | 65 wt.% | 977/1 C | 862.7/100/1 C | [34] |
| S/PANI-coated KB (SPKB) | 57 wt.% | 1338/0.1 C | 675/200/0.1 C | [35] |
| S/PANI-C(SPC) | 2.5 mg cm−2 | 1150/0.2 C | 732/100/0.2 C | [36] |
| C-S@PANI | 40 wt.% | 1453/0.1 C | 948/200/0.1 C 922/200/0.1 C/50 °C 581/200/0.1 C/0 °C | [37] |
| Hollow PANI sphere@S | 62 wt.% | 1392.7/0.2 C | 602/1000/0.5 C | [38] |
| hPANIs@ S | N/A | 761.8/170 mA g−1 | 601.9/100/170 mA g−1 | [39] |
| MWCNT-PANI-G | 68 wt.% | 1290/0.2 C | 784/100/0.2 C | [40] |
| HCNF@PANI-S | 74.4 wt.% | 960/0.5 C | 535/200/0.5 C | [41] |
| Sulfur-PANI-GNRs (SPGs) | N/A | 673/0.4 C | 514/400/0.4 C | [42] |
| nanoS@PANI/G | N/A | 1625/0.1 C | 600/100/0.1 C | [43] |
| PEDOT/GO@S | 66.2 wt.% | 1195.7/0.5 C | 800.2/200/0.5 C | [45] |
| S@h-P | N/A | 341/1 A g−1 | 312/300/1 A g−1 | [46] |
| SPANI | 65 wt.% | N/A | 734/200/0.3 C 600/200/0.6 C 500/200/1 C | [44] |
| PANINF/MWCNT coated separator | N/A | 1020/0.2 C 867/0.5 C 791/1 C | 709/100/0.2 C 641/100/0.5 C 612/100/1 C | [47] |
| PANI-printed on S cathode | N/A | 935/1 C | 901.3/200/1 C | [48] |
| NPGO-S | 3.3 mg cm−2 | 1114/0.2 C 953/1 C | 857.8/100/0.2 C | [49] |
| Composite | Sulfur Content | Capacity (mAh g−1/rate) | Cycling Performance (mAh g−1/cycles/rate) | Ref. |
|---|---|---|---|---|
| PPy@S | N/A | 1200/0.2 C | 913/50/0.2 C | [58] |
| PPy coated S | 61.9 wt.% | 1039/0.1 C | 613/50/0.1 C | [59] |
| PO43− doped PPy coated nano-S | N/A | 1142/0.1 C | 742.3/100/0.1 C | [60] |
| S@PPy/GS | N/A | 1040/0.1 C | 537.8/200/0.2 C | [61] |
| S/PPy-MnO2 | N/A | 1420/0.2 C | 985/200/0.2 C | [62] |
| PPy@S@PPy | 65.6 wt.% | 801/50 mA g−1 | 554/50/50 mA g−1 | [63] |
| Tubular carbon@S@PPy | N/A | 1111/335 mA g−1 | 731/100/335 mA g−1 | [64] |
| S-CNT-PPy | N/A | 1240/50 mA g−1 | 600/40/50 mA g−1 | [65] |
| MWCNTs@S@PPy | N/A | 1517/200 mA g−1 | 917/60/200 mA g−1 | [66] |
| PPY/PEG-S/A-CNT | N/A | 1355/0.1 C | 924/100/0.1 C | [67] |
| GCS@PPY | N/A | 470/3 C | 376/400/3 C | [68] |
| PPy@CMK-8/S | 53.7 wt.% | 1099/0.2 C | 860/100/0.2 C | [70] |
| S-PPy physical mixing | 40 wt.% | 1222 | 570/20 | [71] |
| S/PPyA | N/A | 1285 | 866/40 | [72] |
| S/T-HSSP | 58.4 wt.% | 1563.3/0.2 C | 892.4/200/0.2 C | [73] |
| S/PPy-MWCNT(25 wt.% PPy) | 49 wt.% | 1275/0.1 mA cm−2 | 725.8/100/0.1 mA cm−2 | [74] |
| rGO/PPy/S | 69.43 wt.% | 991.5/1 C 537.4/5 C | 626.7/400/1 C 442.1/400/5 C | [75] |
| Nano-S/PPy/GNS | N/A | 1415.7/0.1 C | 641.5/40/0.1 C | [76] |
| S/PY-GF | 6.2mg cm−2 | 1220/0.2 C 985.8/0.5 C | 797.7/100/0.5 C | [77] |
| S-PPY(ball-milling) | 49 wt.% | 1178/200 mA g−1 | 675/150/200 mA g−1 | [78] |
| S/PPy | N/A | 931/0.1 C | 502.7/100/0.1 C | [79] |
| S/Ketjen black | N/A | 1110.4/0.5 C | 801.6/300/0.5 C | [80] |
| S/Ketjen black | 2.5~3mg cm−2 | 1102/0.5 C | 712/300/0.5 C | [81] |
| CMK-8/S | N/A | 719/0.2 C | 703/300/1 C 533/300/2 C | [83] |
| S-MIEC | 75 wt.% | 968/0.1 C | 500/50/1 C | [82] |
| PPy/S@PPy | 1.4mg cm−2 | 1064/0.1 C | 848/20/0.1 C | [85] |
| Composite | Sulfur Content | Capacity (mAh g−1/rate) | Cycling Performance (mAh g−1/cycles/rate) | Ref. |
|---|---|---|---|---|
| Nano-S@PEDOT | 72 wt.% | 1117/400 mA g−1 | 930/50/400 mA g−1 | [89] |
| S/PEDOT:PSS | N/A | 1100/0.1 C | 565.7/50/0.2 C | [90] |
| PEDOT:PSS-coated CMK3/S | N/A | 1140/0.2 C | 969/100/0.2 C | [92] |
| Graphene and PEDOT:PSS coated nano-S (SGP) | N/A | 1432 Ah L−1/0.1 C 1038 Ah L−1/1 C | 806/500/1 C | [93] |
| Biomolecule-doped PEDOT:PSS coated MIL-101/S (BPCS) | 57.884 wt.% | 1567.74/0.1 C | 606.62/192/0.1 C | [94] |
| S@Na2Fe[Fe(CN)6)]@PEDOT | 82 wt.% | 1291/0.1 C 683/5 C | 1101/100/0.1 C 544/200/5 C | [95] |
| PEDOT-co-PEG coated sulfur (1 wt.% polymer) | N/A | 1619/0.2 C | 1002/100/0.2 C | [96] |
| S/MWCNTols/PEDOP | 70 wt.% | 1611/0.1 C | 624/200/0.1 C | [98] |
| S@PEDOT/MnO2 | 87 wt.% | 1150/0.2 C | 827/200/0.2 C 545/200/0.5 C | [91] |
| S3BT copolymer | 70 wt.% | 1362/0.1 C | 682/500/1 C | [100] |
| S-PMAT copolymer | 1.5mg cm−2 | 1240/0.1 C 600/5 C | 495/1000/2 C | [101] |
| PEDOT:PSS-CNT interlayer | 42 wt.% | 921/0.5 C | 653/200/0.5 C | [102] |
| PEODT:PSS-coated S cathode | 0.75~0.98 mg cm−2 | 1189/0.1 C | 790/50/0.1 C | [104] |
| PEDOT binder/commercial sulfur/PEGDME | 50 wt.% | 850 | 578/100 | [105] |
| PANI | PPy | PTh and PEDOT | |
|---|---|---|---|
| Coating layer | Most works | Most works | Most works |
| Conductive host | Most works | Most works | No works |
| Separator modifier | Few works | Few works | No works |
| Functional interlayer | Few works | Few works | Few works |
| Sulfur-containing copolymer | Few works | No works | Few works |
| Binder | No work | Few works | Few works |
| Current collector | No work | Few works | No works |
| Redox mediator | One work | No work | No work |
| Advantages | Low cost, facile preparation, widely used | Facile preparation, widely used, high conductivity | Commercialized, easy to fabricate |
| Shortcomings | Poor conductivity | Expensive | Hard to synthesize |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, X.; Liu, Y.; Li, Y.; Wang, X.; Fu, J.; Wang, X. Application Progress of Polyaniline, Polypyrrole and Polythiophene in Lithium-Sulfur Batteries. Polymers 2020, 12, 331. https://doi.org/10.3390/polym12020331
Hong X, Liu Y, Li Y, Wang X, Fu J, Wang X. Application Progress of Polyaniline, Polypyrrole and Polythiophene in Lithium-Sulfur Batteries. Polymers. 2020; 12(2):331. https://doi.org/10.3390/polym12020331
Chicago/Turabian StyleHong, Xiaodong, Yue Liu, Yang Li, Xu Wang, Jiawei Fu, and Xuelei Wang. 2020. "Application Progress of Polyaniline, Polypyrrole and Polythiophene in Lithium-Sulfur Batteries" Polymers 12, no. 2: 331. https://doi.org/10.3390/polym12020331
APA StyleHong, X., Liu, Y., Li, Y., Wang, X., Fu, J., & Wang, X. (2020). Application Progress of Polyaniline, Polypyrrole and Polythiophene in Lithium-Sulfur Batteries. Polymers, 12(2), 331. https://doi.org/10.3390/polym12020331