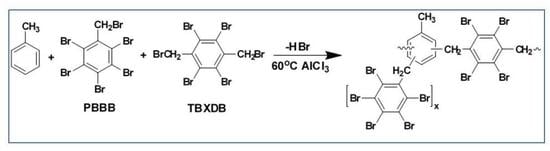
Alkylation of Aromatic Compounds with Pentabromobenzyl Bromide and Tetrabromoxylene Dibromide as a New Route to High Molecular Weight Brominated Flame Retardants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Analysis
2.3. Gel Permeation Chromatography (GPC)
2.4. Infrared Spectroscopy (FTIR)
2.5. Thermal Analysis
2.6. Ingredients, Compounding, and Molding
2.7. Flammability Test
2.8. Mechanical Properties
2.9. Heat Distortion Temperature (HDT)
2.10. Melt Flow Index (MFI)
2.11. Synthesis
2.11.1. Alkylation of Toluene with PBBB (T-PBBB)

2.11.2. Alkylation of Diphenylethane with PBBB (DPE-PBBB)

2.11.3. Alkylation of Toluene with PBBB and TBXDB (T-PBBB-TBXDB)

2.11.4. Alkylation of Diphenylethane with PBBB and TBXDB (DPE-PBBB-TBXDB)

2.11.5. Alkylation of PPE with PBBB (PPE-PBBB)

2.11.6. Alkylation of PS with PBBB (PS-PBBB)

3. Results and Discussion
3.1. Thermogravimetry
3.2. Combustion
3.2.1. Polypropylene Copolymer
3.2.2. ABS and HIPS
3.2.3. Polyamide 6.6
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Williams, C. Polymeric flame retardants to replace DecaBDE. Speciality Chemicals Magazines, September 2012; 28–30. [Google Scholar]
- Mapleston, P. Flame retardants trend to polymerics. Compounding Word, December 2016; 15–30. [Google Scholar]
- Borgnes, D.; Rikhein, B. Emission Measurements during Incineration of Waste Containing Bromine; Kjelfireningen Nork Energi Report No. 25391; Nork Energi: Oslo, Norway, 2004. [Google Scholar]
- Tange, L.; Drohmann, D. Environmental issues related to end-of-life options of plastics containing brominated flame retardants. Fire Mater. 2004, 28, 403–410. [Google Scholar] [CrossRef]
- Tange, L.; Van Houwelingen, J.A.; Peeters, J.R.; Vanegas, P. Recycling of flame retardant plastics from WEEE, technical and environmental challenges. Adv. Prod. Eng. Manag. 2013, 8, 63–144. [Google Scholar] [CrossRef] [Green Version]
- Peetersa, J.R.; Vanegas, P.; Tange, L.; Van Houwelingen, J.; Duflou, J.R. Closed loop recycling of plastics containing flame retardants. Resour. Conserv. Recycl. 2014, 84, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Imai, T. Comparative recyclability of flame retarded plastics. In Proceedings of the International Fire Safety Conferene, FRCA, San Francisco, CA, USA, 11–14 March 2001; pp. 63–86. [Google Scholar]
- Imai, T.; Hamm, S.; Rothenbacher, K.P. Comparison of recyclability of flame retarded plastics. Environ. Sci. Technol. 2003, 37, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Levchik, S.; Georlette, P.; Bar-Yaakov, Y.; Finberg, I.; Hirschsohn, Y. Polymeric flame retardants. In Proceedings of the Conference Recent Advances in Flame Retardancy of Polymeric Materials, BCC, Stamford, CT, USA, 24–26 May 2010. [Google Scholar]
- King, B.; Stobby, W.G.; Murray, D.J.; Worku, A.Z.; Beulich, I.; Tinetti, S.M.; Hahn, S.F.; Drumright, R.E. Brominated Butadiene/Vinyl Aromatic Copolymers, Blends of Such Copolymers with Vinylaromatic Polymer, and Foams Fromed from Such Blends. European Patent 1,957,544, 10 January 2010. to Dow Chemicals. [Google Scholar]
- Beach, M.; King, B.; Beulich, I.; Morgan, T.; Stobby, B.; Kram, S.; Hull, J.; Lukas, C.; Kiefer, J.; Leng, R.; et al. New class of brominated polymeric flame retardants. In Proceedings of the Conference on Recent Advances in Flame Retardancy of Polymeric Materials, BCC, Stamford, CT, USA, 23–25 May 2011. [Google Scholar]
- Layman, W.J.; Kolich, C.H.; Mack, A.G.; Anderson, S.A.; McCarney, J.P.; Morice, J.; Ge, Z.; Wang, J. Bromination of Telomer Mixtures Derived from Toluene and Styrene. U.S. Patent 8,642,821, 4 February 2014. to Albemarle. [Google Scholar]
- Timberlake, L.D.; Fielding, W.R.; Mathur, S.; Hanson, M.V. Brominated Flame Retardant. U.S. Patent 7,550,551, 23 June 2009. to Chemtura. [Google Scholar]
- King, B.A.; Worku, A.; Stobby, W.G. Brominated Polymers as Flame Additives and Polymer Systems Containing Same. European Patent 2,247,664, 2 June 2012. to Dow Chemicals. [Google Scholar]
- Yashiki, K.; Sudo, A. Bromine-Containing Polymers and Methods for Producing the Same. U.S. Patent 10,442,876, 15 October 2019. to Manac. [Google Scholar]
- Campbell, E.J.; Czaplewski, S.K.; Kobilka, B.M.; Wertz, J.T. Flame Retardant Polycaprolactone. U.S. Patent 10,308,875, 4 June 2019. to IBM. [Google Scholar]
- Peled, M.; Kornberg, N. Process for the Preparation of Poly-(halobenzyl acrylate). U.S. Patent 6,028,156, 22 February 2000. to Bromine Compounds. [Google Scholar]
- Kornberg, N.; Adda, M.; Peled, M. Synthesis of Polyhalogenated Halomethyl Compounds. U.S. Patent 7,601,774, 13 October 2009. to Bromine Compounds. [Google Scholar]
- Colthup, N.R.; Daly, L.H.; Wiberly, S.E. Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Lieberman, R.; Stewart, C. Polypropylene Polymers. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2002; Volume 11, pp. 287–358. [Google Scholar]
- Bar Yaakov, Y.; Utevski, L.; Reyes, J.; Georlette, P.; Bron, S.; Lopez-Cuesta, J.M. Existing fire retardant systems for polypropylene and its copolymers and new developments. In Flame Retardants 2000; Interscience Communications: London, UK, 2000; pp. 87–97. [Google Scholar]
- Hini, S.; Reznick, G.; Bar Yaakov, Y.; Georlette, P.; Geran, T.; Squires, G. A new dimension in fire safety. In Proceedings of the Conference on Recent Advances in Flame Retardancy of Polymeric Materials, Stamford, CT, USA, 3–5 June 2002. [Google Scholar]
- Georlette, P.; Simons, J.; Costa, L. Halogen-containing fire-retardant compounds. In Fire Retardancy of Polymeric Materials; Grand, A.F., Wilkie, C.A., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 246–284. [Google Scholar]
- Horak, Z.; Rosik, L. Modification of flammability characteristics. In Styrene-Based Plastics and Their Modification; Svec, P., Rosik, L., Horak, Z., Vecerka, F., Eds.; Ellis Horwood: New York, NY, USA, 1990; pp. 277–299. [Google Scholar]
- Hirschler, M.M. Chemical Aspects of Thermal Decomposition of Polymeric Materials. In Fire Retardancy of Polymeric Materials; Grand, A.F., Wilkie, C.A., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 27–79. [Google Scholar]
- Hochberg, A.; Glogovsky, J. Key properties of FR engineering thermoplastics for demanding applications. In Proceedings of the Society of Plastic Engineers ANTEC Conference, Boston, MA, USA, 7–11 May 1995; pp. 3518–3526. [Google Scholar]
- Crosby, J.M.; Talley, K.L. Designing in reinforced flame-retardant plastics. Mod. Plast. 1982, 59, 66–70. [Google Scholar]







| Component | Trade Name and Manufacture | Function |
|---|---|---|
| Impact-modified polypropylene (PP) | Capilene® SL-50 (Caol) | Polymer matrix |
| Polyamide 6.6 (contains nucleating agent, mold release agent, and lubricant) (PA 6.6) | Aculon® S 223D (DSM) | Polymer matrix |
| High-impact polystyrene (HIPS) | StyronTM 1200 (Dow) | Polymer matrix |
| Acrylonitrile–butadiene–styrene copolymer (ABS) | ABS MagnumTM 3404 (Styron) | Polymer matrix |
| Decabromodiphenylethane | FR-1410 (ICL-IP) | Reference flame retardant |
| Poly(pentabromobenzyl acrylate) | FR-1025 (ICL-IP) | Reference flame retardant |
| Brominated epoxy polymer | F-3014 (ICL-IP) | Reference flame retardant |
| Tribromophenol-end-capped brominated epoxy polymer | F-3020 (ICL-IP) | Reference flame retardant |
| Brominated polystyrene | FR-803P (ICL-IP) | Reference flame retardant |
| Antimony trioxide masterbatch (MB) containing 80 wt.% Sb2O3 | FR00112 (Kafrit) | Flame retardant (FR) synergist |
| Polytetrafluoroethylene (PTFE) | Hostaflon® 2017 (Dyneon) | Anti-dripping agent |
| Styrene–butadiene–styrene block copolymer (SBS) | SBS 501S (LG Chem) | Impact modifier |
| Talc | Lotalc | Filler |
| Masterbatch of talc, 60 wt.% | Talc MB (Kafrit) | Filler |
| Glass fibers | GF ChopVantage® 3660 (PPG) | Filler |
| Blend of tris(2,4-ditert-butylphenyl)phosphite and pentaerythritol tetrakis [3-[3,5-di-tert-butyl-4-hydroxyphenyl]propionate] (50:50) | Irganox® B 225 (BASF) | Antioxidant and heat stabilizer |
| Multifunctional nitrogen-containing hindered phenol | Acrawax® C (Lonza) | Antioxidant and heat stabilizer |
| N,N′ ethylene bis stearamide | Irganox® B1171 (BASF) | Lubricant |
| Calcium stearate | Ca-stearate | Lubricant |
| Flame Retardant | Initial Decomposition | Main Step of Weight Loss | Solid Residue | ||
|---|---|---|---|---|---|
| T5%, °C | Tmax, °C | Tend, °C | Wt. loss, % | Wt.% | |
| T-PBBB | 370 | 410 | 450 | 35 | 19 |
| DBE-PBBB | 375 | 405 | 455 | 35 | 21 |
| T-PBBB-TBXDP | 375 | 410 | 475 | 55 | 26 |
| DBE-PBBB-TBXDP | 360 | 400 | 480 | 50 | 24 |
| PPE-TBBB | 370 | 375 | 390 | 20 | 23 |
| PS-TBBB | 360 | 410 | 435 | 50 | 19 |
| * Composition, wt.% | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Flame Retardant | FR-1410 | FR-1025 | DPE-PBBB | DPE-PBBB-TBXDB | PPE-PBBB | PS-PBBB | FR-1025 | DPE-PBBB | FR-1025 | PS-PBBB |
| Impact-modified PP | 68.5 | 54.6 | 56.7 | 54.7 | 54.6 | 59.7 | 51.9 | 53.3 | 50.3 | 49.5 |
| Flame retardant | 25.6 | 32.4 | 29.3 | 30.7 | 30.9 | 28 | 25.4 | 24.0 | 25.3 | 26.1 |
| Talc or talc MB | 15.0 | 15.0 | 16.7 (10) | 16.7 (10) | ||||||
| Antimony trioxide MB | 13.1 | 12.8 | 13.8 | 14.4 | 14.4 | 12.1 | 7.5 | 7.5 | 7.5 | 7.5 |
| Br content, % calculated | 21.0 | 23.0 | 22.0 | 23.0 | 23.0 | 19.3 | 18.0 | 18.0 | 18.0 | 18.0 |
| Sb2O3, % calculated | 10.5 | 11.5 | 11.0 | 11.5 | 11.5 | 9.7 | 6.0 | 6.0 | 6.0 | 6.0 |
| Br/Sb2O3 ratio | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| UL-94 rating | V-0 | V-0 | V-0 | V-0 | V-0 | V-0 | V-0 | V-0 | V-0 | V-0 |
| Impact strength (J/m) | 72 | 31 | 27 | 35 | 32 | 33 | 26 | 28 | 35 | 34 |
| Tensile strength (MPa) | 21.2 | 24.6 | 17.2 | 17.6 | 17.0 | 18.9 | 22.3 | 18.0 | 20.4 | 18.6 |
| Elongation at break (%) | 127 | 6.1 | 64 | 27 | 102 | 47 | 6.9 | 27 | 27 | 57 |
| Tensile modulus (MPa) | 1550 | 1800 | 1490 | 1660 | 1450 | 1620 | 2080 | 2060 | 2170 | 1790 |
| HDT (°C) | 55 | 84 | 63 | 58 | 57 | 61 | 77 | 67 | 74 | 61 |
| MFI (g/10 min) | 5.3 | 12.5 | 4.1 | 3.8 | 5.9 | 3.8 | 6.2 | 3.5 | 5.0 | 2.9 |
| * Composition, wt.% | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Flame Retardant | FR-1410 | F-3020 | DPE-PBBB | DPE-PBBB | DPE-PBBB-TBXDB | PPE-PBBB | PPE-PBBB | PPE-PBBB |
| ABS | 82.5 | 76.8 | 81.4 | 80.4 | 81.4 | 81.4 | 81.7 | 80.5 |
| Flame retardant | 12.2 | 17.9 | 13.3 | 17.4 | 13.3 | 13.3 | 15.3 | 17.3 |
| Antimony trioxide MB | 5.0 | 5.0 | 5.0 | 1.9 | 5.0 | 5.0 | 2.6 | 1.9 |
| Br content, % calculated | 10.0 | 10.0 | 10.0 | 13.0 | 10.0 | 10 | 11.5 | 13.0 |
| Sb2O3, % calculated | 4.0 | 4.0 | 4.0 | 1.5 | 4.0 | 4.0 | 2.1 | 1.5 |
| Bromine/Sb2O3 ratio | 2.5 | 2.5 | 2.5 | 8.7 | 2.5 | 2.5 | 5.5 | 8.7 |
| UL-94 rating | V-0 | V-0 | V-0 | V-0 | V-0 | V-0 | V-0 | V-0 |
| Impact strength (J/m) | 128 | 113 | 71 | 104 | 69 | 92 | 78 | 74 |
| Tensile strength (MPa) | 40 | 43 | 37 | 39 | 38 | 38 | 38 | 37 |
| Elongation at break (%) | 16 | 3 | 7 | 13 | 11 | 6 | 6 | 6 |
| Tensile modulus (MPa) | 2400 | 2330 | 2330 | 2160 | 2330 | 2080 | 2100 | 2070 |
| HDT (°C) | 78 | 76 | 77 | 78 | 78 | 77 | 78 | 79 |
| MFI (g/10 min) | 8.8 | 22.7 | 15.3 | 11.7 | 13.4 | 12.4 | 12.3 | 10.6 |
| * Composition, wt.% | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Flame Retardant | F-3014 | F-3014 | F-3014 | DPE-PBBB-TBXDB | DPE-PBBB-TBXDB | DPE-PBBB-TBXDB |
| HIPS | 78.0 | 78.8 | 76.1 | 82.2 | 83.3 | 81.5 |
| Flame retardant | 16.7 | 18.3 | 21.7 | 12.5 | 13.8 | 16.3 |
| Antimony trioxide MB | 5.0 | 2.6 | 1.9 | 5.0 | 2.6 | 1.9 |
| Br content, % calculated | 10 | 11 | 13 | 10 | 11 | 13 |
| Sb2O3, % calculated | 4.0 | 2.1 | 1.5 | 4.0 | 2.1 | 1.5 |
| Bromine/Sb2O3 ratio | 2.5 | 5.2 | 8.7 | 2.5 | 5.2 | 8.7 |
| UL-94 rating | V-1 | V-0 | V-0 | V-1 | V-0 | V-0 |
| Impact strength (J/m) | 67 | 70 | 67 | 43 | 43 | 38 |
| Tensile strength (MPa) | 26 | 25 | 25 | 24 | 25 | 23 |
| Elongation at break (%) | 40 | 57 | 47 | 17 | 15 | 11 |
| Tensile modulus (MPa) | 1980 | 1920 | 1910 | 2100 | 2070 | 2050 |
| HDT (°C) | 65 | 66 | 65 | 71 | 72 | 72 |
| MFI (g/10 min) | 14.4 | 13.8 | 14.2 | 4.2 | 4.0 | 4.2 |
| * Composition, wt.% | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Flame Retardant | FR-803P | T-PBBB | DPE-PBBB | PPE-PBBB | PS-PBBB |
| PA 6.6 | 43.5 | 46.3 | 51.3 | 45.8 | 44.1 |
| Glass fiber | 30 | 30 | 30 | 30 | 30 |
| Flame retardant | 19.7 | 16.9 | 13.3 | 17.4 | 19.1 |
| Antimony trioxide MB | 6.2 | 6.2 | 4.8 | 6.2 | 6.2 |
| Br (calculated) | 13 | 13 | 10 | 13 | 13 |
| Sb2O3 (calculated) | 4.8 | 5.0 | 3.8 | 5.0 | 5.0 |
| Br/Sb2O3 (calculated) | 2.7 | 2.6 | 2.6 | 2.6 | 2.6 |
| UL-94 rating | V-0 | V-0 | V-0 | V-0 | V-0 |
| Izod Impact (J/m) | 111 | 104 | 103 | 104 | 97 |
| Tensile strength (MPa) | 143 | 150 | 150 | 135 | 137 |
| Elongation at break (%) | 3.3 | 4.2 | 4.7 | 4.3 | 3.5 |
| Tensile modulus (MPa) | 10050 | 10750 | 9950 | 8630 | 10450 |
| HDT (°C) | 225 | 226 | 231 | 228 | 216 |
| MFI (g/10 min) | 7 | 11 | 29 | 13 | 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelmont, M.; Yuzefovitch, M.; Yoffe, D.; Eden, E.; Levchik, S. Alkylation of Aromatic Compounds with Pentabromobenzyl Bromide and Tetrabromoxylene Dibromide as a New Route to High Molecular Weight Brominated Flame Retardants. Polymers 2020, 12, 352. https://doi.org/10.3390/polym12020352
Gelmont M, Yuzefovitch M, Yoffe D, Eden E, Levchik S. Alkylation of Aromatic Compounds with Pentabromobenzyl Bromide and Tetrabromoxylene Dibromide as a New Route to High Molecular Weight Brominated Flame Retardants. Polymers. 2020; 12(2):352. https://doi.org/10.3390/polym12020352
Chicago/Turabian StyleGelmont, Mark, Michael Yuzefovitch, David Yoffe, Eyal Eden, and Sergei Levchik. 2020. "Alkylation of Aromatic Compounds with Pentabromobenzyl Bromide and Tetrabromoxylene Dibromide as a New Route to High Molecular Weight Brominated Flame Retardants" Polymers 12, no. 2: 352. https://doi.org/10.3390/polym12020352
APA StyleGelmont, M., Yuzefovitch, M., Yoffe, D., Eden, E., & Levchik, S. (2020). Alkylation of Aromatic Compounds with Pentabromobenzyl Bromide and Tetrabromoxylene Dibromide as a New Route to High Molecular Weight Brominated Flame Retardants. Polymers, 12(2), 352. https://doi.org/10.3390/polym12020352