Main Chain–Type Block Copolymers through Atom Transfer Radical Polymerization from Double-Decker–Shaped Polyhedral Oligomeric Silsesquioxane Hybrids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bis(4-Vinylbenzyl chloride) Double-Decker Silsesquioxane (VBC-DDSQ-VBC)
2.3. PS-DDSQ-PS
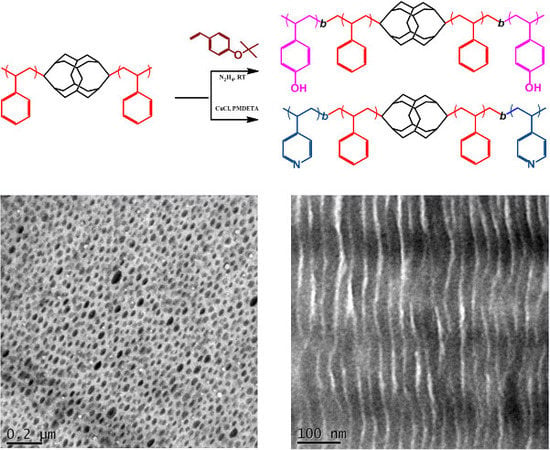
2.4. P4VP-b-PS-DDSQ-PS-b-P4VP
2.5. PtBuOS-b-PS-DDSQ-PS-b-PtBuOS and PVPh-b-PS-DDSQ-PS-b-PVPh
2.6. Characterization
3. Results
3.1. Synthesis of the Homopolymer PS-DDSQ-PS
3.2. Synthesis of PtBuOS-b-PS-DDSQ-PS-b-PtBuOS and PVPh-b-PS-DDSQ-PS-b-PVPh
3.3. Synthesis of P4VP-b-PS-DDSQ-PS-b-P4VP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guiod, A.R.; Vandermeulen, W.M.; Klok, V.H. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar]
- Vu, D.T.; Chiu, H.W.; Nababan, R.; Le, Q.M.; Kuo, S.W.; Chau, L.K.; Ting, C.C.; Kan, H.C.; Hsu, C.C. Enhancing upconversion luminescence emission of rare earth nanophosphors in aqueous solution with thousands fold enhancement factor by low refractive index resonant waveguide grating. ACS Photonics 2018, 5, 3263–3271. [Google Scholar] [CrossRef]
- Lin, E.L.; Hsu, W.L.; Chiang, Y.W. Trapping structural coloration by a bioinspired gyroid microstructure in solid state. ACS Nano 2018, 12, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Jenekhe, S.A.; Chen, X.L. Self-assembly of ordered microporous materials from rod-coil block copolymers. Science 1999, 283, 372–375. [Google Scholar] [CrossRef]
- Fuller, E.G.; Sun, H.; Dhavalikar, R.D.; Unni, M.; Scheutz, G.M.; Sumerlin, B.S.; Rinaldi, C. Externally Triggered Heat and Drug Release from Magnetically Controlled Nanocarriers. ACS Appl. Polym. Mater. 2019, 1, 211–220. [Google Scholar] [CrossRef]
- Jiang, M.; Xie, H. Miscibility and morphology in block copolymer/homopolymer blends. Prog. Polym. Sci. 1991, 16, 977–1026. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Pearce, E.M.; Kwei, T.K. Binary and ternary blends of polystyrene-block-poly(p-hydroxystyrene). Macromolecules 1997, 30, 7119–7126. [Google Scholar] [CrossRef]
- Han, Y.K.; Pearce, E.M.; Kwei, T.K. Poly(styrene-b-vinylphenyldimethylsilanol) and its blends with homopolymers. Macromolecules 2000, 33, 1321–1329. [Google Scholar] [CrossRef]
- Shih, R.S.; Kuo, S.W.; Chang, F.C. Thermal and mechanical properties of microcellular thermoplastic SBS/PS/SBR blend: Effect of crosslinking. Polymer 2011, 52, 752–759. [Google Scholar] [CrossRef]
- Dobrosielska, K.; Wakao, S.; Takano, A.; Matsushita, Y. Nanophase-separated structures of AB block copolymer/C homopolymer blends with complementary hydrogen-bonding interactions. Macromolecules 2008, 41, 7695–7698. [Google Scholar] [CrossRef]
- Dehghan, A.; Shi, A.C. Modeling hydrogen bonding in diblock copolymer/homopolymer blends. Macromolecules 2013, 46, 5796–5805. [Google Scholar] [CrossRef]
- Miyase, H.; Asai, Y.; Takano, A.; Matsushita, Y. Kaleidoscopic tiling patterns with large unit cells from ABC star-shaped terpolymer/diblock copolymer blends with hydrogen bonding interaction. Macromolecules 2017, 50, 979–986. [Google Scholar] [CrossRef]
- Tsai, C.C.; Gan, Z.; Chen, T.; Kuo, S.W. Competitive Hydrogen Bonding Interactions Influence the Secondary and Hierarchical Self-Assembled Structures of Polypeptide-Based Triblock Copolymers. Macromolecules 2018, 51, 3017–3029. [Google Scholar] [CrossRef]
- Tseng, T.C.; Kuo, S.W. Hydrogen-Bonding Strength Influences Hierarchical Self-Assembled Structures in Unusual Miscible/Immiscible Diblock Copolymer Blends. Macromolecules 2018, 51, 6451–6459. [Google Scholar] [CrossRef]
- Tsou, C.T.; Kuo, S.W. Competing Hydrogen Bonding Interaction Creates Hierarchically Ordered Self-Assembled Structures of PMMA-b-P4VP/PVPh-b-PS Mixtures. Macromolecules 2019, 52, 8374–8383. [Google Scholar] [CrossRef]
- Tseng, T.C.; Kuo, S.W. Hydrogen bonding induces unusual self-assembled structures from mixtures of two miscible disordered diblock copolymers. Eur. Polym. J. 2019, 116, 361–369. [Google Scholar] [CrossRef]
- Su, W.C.; Tsai, F.C.; Huang, C.F.; Dai, L.; Kuo, S.W. Flexible Epoxy Resins Formed by Blending with the Diblock Copolymer PEO-b-PCL and Using a Hydrogen-Bonding Benzoxazine as the Curing Agent. Polymers 2019, 11, 201. [Google Scholar] [CrossRef] [Green Version]
- Tseng, T.C.; Kuo, S.W. Hierarchical Self-Assembled Structures from Diblock Copolymer Mixtures by Competitive Hydrogen Bonding Strength. Molecules 2018, 23, 2242. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guo, S.; Fu, S.; Zhao, Y. A Near-Infrared Light-Responsive Hybrid Hydrogel Based on UCST Triblock Copolymer and Gold Nanorods. Polymers 2017, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Hoheisel, T.N.; Hur, K.; Wiesner, U.B. Block copolymer-nanoparticle hybrid self-assembly. Prog. Polym. Sci. 2015, 40, 3–32. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, B.; Alexandridis, P. Block copolymer–nanoparticle composites: Structure, functional properties, and processing. Prog. Polym. Sci. 2015, 40, 33–62. [Google Scholar] [CrossRef]
- Wang, H.S.; Khan, A.; Choe, Y.; Huh, J.; Bang, J.; Stefik, M.; Guldin, S.; Vignolini, S.; Wiesner, U.B.; Steinere, U. Block copolymer self-assembly for nanophotonics. Chem. Soc. Rev. 2015, 44, 5076–5091. [Google Scholar]
- Mable, C.J.; Gibson, R.R.; Prevost, S.; McKenzie, B.E.; Mykhaylyk, O.O.; Armes, S.P. Loading of Silica Nanoparticles in Block Copolymer Vesicles during Polymerization-Induced Self-Assembly: Encapsulation Efficiency and Thermally Triggered Release. J. Am. Chem. Soc. 2015, 137, 16098–16108. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Kuo, S.W. Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.K.; Tsai, F.C.; Ma, C.C.; Wang, M.L.; Kuo, S.W. Using Methacryl-Polyhedral Oligomeric Silsesquioxane as the Thermal Stabilizer and Plasticizer in Poly (vinyl chloride) Nanocomposites. Polymers 2019, 11, 1711. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.G.; Kuo, S.W. Functional Silica and Carbon Nanocomposites Based on Polybenzoxazines. Macromol. Chem. Phys. 2019, 220, 1800306. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.; Lin, R.C.; Tseng, S.M.; Kuo, S.W. Minimizing the Strong Screening Effect of Polyhedral Oligomeric Silsesquioxane Nanoparticles in Hydrogen-Bonded Random Copolymers. Polymers 2018, 10, 303. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Shah, S.M.; Hussain, H. Amphiphilic tadpole-shaped POSS-poly(glycerol methacrylate) hybrid polymers: Synthesis and self-assembly. J. Polym. Res. 2019, 26, 4. [Google Scholar] [CrossRef]
- Lu, Y.S.; Kuo, S.W. Functional groups on POSS nanoparticles influence the self-assembled structures of diblock copolymer composites. RSC Adv. 2014, 4, 34849–34859. [Google Scholar] [CrossRef]
- Lu, Y.S.; Yu, C.Y.; Lin, Y.C.; Kuo, S.W. Hydrogen Bonding Strength of Diblock Copolymers Affects the Self-Assembled Structures with Octa-Functionalized Phenol POSS Nanoparticles. Soft Matt. 2016, 12, 2288–2300. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, S.W. Phenolic Functionality of Polyhedral Oligomeric Silsesquioxane Nanoparticles Affects Self-Assembly Supramolecular Structures of Block Copolymer Hybrid Complexes. Ind. Eng. Chem. Res. 2018, 57, 2546–2559. [Google Scholar] [CrossRef]
- Wu, Y.R.; Wu, Y.C.; Kuo, S.W. Transforming the Self-Assembled Structures of Diblock Copolymer/POSS Nanoparticle Composites through Complementary Multiple Hydrogen Bonding Interactions. Macromol. Chem. Phys. 2013, 214, 1496–1503. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, M.; Su, H.; Zhang, S.; Yue, K.; Dong, X.H.; Li, X.; Liu, H.; Zhang, S.; Wesdemiotis, C.; et al. Toward Controlled Hierarchical Heterogeneities in Giant Molecules with Precisely Arranged Nano Building Blocks. ACS Cent. Sci. 2016, 2, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Zhong, S.; Li, X.; Tu, Y.; Yang, S.; Horn, R.M.; Ni, C.; Pochan, D.J.; Quirk, R.P.; Wesdemiotis, C.; et al. A Giant Surfactant of Polystyrene−(Carboxylic Acid-Functionalized Polyhedral Oligomeric Silsesquioxane) Amphiphile with Highly Stretched Polystyrene Tails in Micellar Assemblies. J. Am. Chem. Soc. 2010, 132, 16741–16744. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yue, K.; Hsieh, I.F.; Li, Y.; Dong, X.H.; Liu, C.; Xin, Y.; Wang, H.F.; Shi, A.C.; Newkome, G.R.; et al. Giant surfactants provide a versatile platform for sub-10-nm nanostructure engineering. Proc. Natl. Acad. Sci. USA 2013, 110, 10078–10083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.F.; Kuo, S.W.; Lin, F.J.; Huang, W.J.; Wang, C.F.; Chen, W.Y.; Chang, F.C. Influence of PMMA-Chain-End Tethered Polyhedral Oligomeric Silsesquioxanes on the Miscibility and Specific Interaction with Phenolic Blends. Macromolecules 2006, 39, 300–308. [Google Scholar] [CrossRef]
- Chiou, C.W.; Lin, Y.C.; Wang, L.; Hirano, C.; Suzuki, Y.; Hayakawa, T.; Kuo, S.W. Strong Screening Effect of Polyhedral Oligomeric Silsesquioxanes (POSS) Nanoparticles on Hydrogen Bonded Polymer Blends. Polymers 2014, 6, 926–948. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Kuo, S.W. Hierarchical self-assembly and secondary structures of linear polypeptides graft onto POSS in the side chain through click chemistry. Polym. Chem. 2012, 3, 162–171. [Google Scholar] [CrossRef]
- Lin, Y.C.; Kuo, S.W. Self-assembly and secondary structures of linear polypeptides tethered to polyhedral oligomeric silsesquioxane nanoparticles through click chemistry. J. Polym. Sci. Part. A Polym. Chem. 2011, 49, 2127–2137. [Google Scholar] [CrossRef]
- Zhang, W.B.; Sun, B.; Li, H.; Ren, X.; Janoski, J.; Sahoo, S.; Dabney, D.E.; Wesdemiotis, C.; Quirk, R.P.; Cheng, S.Z.D. Synthesis of In-Chain-Functionalized Polystyrene-blockpoly(dimethylsiloxane) Diblock Copolymers by Anionic Polymerization and Hydrosilylation Using Dimethyl-[4-(1-phenylvinyl)phenyl]silane. Macromolecules 2009, 42, 7258–7262. [Google Scholar] [CrossRef]
- Lu, C.H.; Wang, J.H.; Chang, F.C.; Kuo, S.W. Star Block Copolymers through Nitroxide-Mediated Radical Polymerization from Polyhedral Oligomeric Silsesquioxane (POSS) Core. Macromol. Chem. Phys. 2010, 211, 1339–1347. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; He, C. Some recent developments of polyhedral oligomeric silsesquioxane (POSS)-based polymeric materials. J. Mater. Chem. 2011, 21, 2775–2782. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, L.; Zheng, H.; He, B. Synthesis and Drug Release of Star-Shaped Poly(benzyl l-aspartate)-block-poly(ethylene glycol) Copolymers with POSS Cores. Macromol. Biosci. 2014, 14, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Ahmed, M.M.M.; Wang, C.F.; Huang, C.F.; Kuo, S.W. Highly thermally stable mesoporous Poly (cyanate ester) featuring double-decker–shaped polyhedral silsesquioxane framework. Polymer 2019, 185, 121940. [Google Scholar] [CrossRef]
- Chen, W.C.; Kuo, S.W. Ortho-Imide and Allyl Groups Effect on Highly Thermally Stable Polybenzoxazine/Double-Decker-Shaped Polyhedral Silsesquioxane Hybrids. Macromolecules 2018, 51, 9602–9612. [Google Scholar] [CrossRef]
- Liao, Y.T.; Lin, Y.C.; Kuo, S.W. Highly Thermally Stable, Transparent, and Flexible Polybenzoxazine Nanocomposites by Combination of Double-Decker-Shaped Polyhedral Silsesquioxanes and Polydimethylsiloxane. Macromolecules 2017, 50, 5739–5747. [Google Scholar] [CrossRef]
- Kuo, S.W.; Huang, C.F.; Tung, P.H.; Huang, W.J.; Huang, J.M.; Chang, F.C. Synthesis, thermal properties, and specific interactions of high Tg increase in poly (2, 6-dimethyl-1, 4-phenylene oxide)-block-polystyrene copolymers. Polymer 2005, 46, 9348–9361. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Cozzo, G.; Latteri, A.; Recca, A. Synthesis and thermal characterization of new dumbbell shaped POSS/PS nanocomposites: Influence of the symmetrical structure of the nanoparticles on the dispersion/aggregation in the polymer matrix. Polym. Compos. 2015, 36, 1394–1400. [Google Scholar] [CrossRef]











| Sample | PS (Mn) a | PDI b |
|---|---|---|
| 1 h | 4000 | 1.05 |
| 2 h | 4300 | 1.16 |
| 3 h | 5400 | 1.22 |
| 4 h | 7600 | 1.27 |
| 5 h | 11,100 | 1.31 |
| 6 h | 19,000 | 1.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-C.; Tsao, Y.-H.; Wang, C.-F.; Huang, C.-F.; Dai, L.; Chen, T.; Kuo, S.-W. Main Chain–Type Block Copolymers through Atom Transfer Radical Polymerization from Double-Decker–Shaped Polyhedral Oligomeric Silsesquioxane Hybrids. Polymers 2020, 12, 465. https://doi.org/10.3390/polym12020465
Chen W-C, Tsao Y-H, Wang C-F, Huang C-F, Dai L, Chen T, Kuo S-W. Main Chain–Type Block Copolymers through Atom Transfer Radical Polymerization from Double-Decker–Shaped Polyhedral Oligomeric Silsesquioxane Hybrids. Polymers. 2020; 12(2):465. https://doi.org/10.3390/polym12020465
Chicago/Turabian StyleChen, Wei-Cheng, Yu-Hsuan Tsao, Chih-Feng Wang, Chih-Feng Huang, Lizong Dai, Tao Chen, and Shiao-Wei Kuo. 2020. "Main Chain–Type Block Copolymers through Atom Transfer Radical Polymerization from Double-Decker–Shaped Polyhedral Oligomeric Silsesquioxane Hybrids" Polymers 12, no. 2: 465. https://doi.org/10.3390/polym12020465
APA StyleChen, W. -C., Tsao, Y. -H., Wang, C. -F., Huang, C. -F., Dai, L., Chen, T., & Kuo, S. -W. (2020). Main Chain–Type Block Copolymers through Atom Transfer Radical Polymerization from Double-Decker–Shaped Polyhedral Oligomeric Silsesquioxane Hybrids. Polymers, 12(2), 465. https://doi.org/10.3390/polym12020465