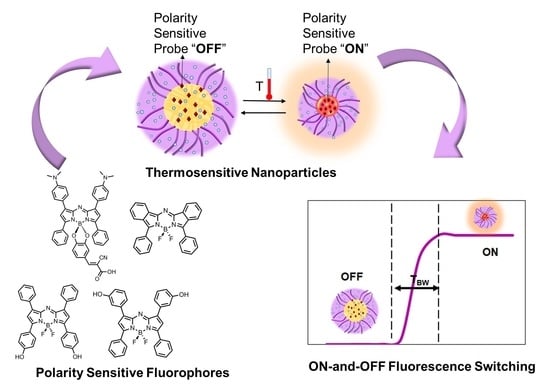
Exploring NIR Aza-BODIPY-Based Polarity Sensitive Probes with ON-and-OFF Fluorescence Switching in Pluronic Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fluorophore Characteristics
2.2. Fluorescence Measurement System
2.3. Fluorescence Emission Intensity and Lifetime Measurement
2.4. Solvents with Different Polarities
2.5. Solvents with Different Viscosities
2.6. Preparation of Fluorophore-Encapsulated Pluronic Nanoparticles
3. Results and Discussion
3.1. Response to Polarity
3.2. Response to Viscosity
3.3. Fluorophore Encapsulation in Pluronic Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Tasior, M.; Murtagh, J.; Frimannsson, D.O.; McDonnell, S.O.; O’Shea, D.F. Water-solubilised BF2-chelated tetraarylazadipyrromethenes. Org. Biomol. Chem. 2010, 8, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Boil. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Cao, J.; He, Y.; Yang, J.H.; Kim, T.; Peng, X.; Kim, J.S. Macro-/micro-environment-sensitive chemosensing and biological imaging. Chem. Soc. Rev. 2014, 43, 4563–4601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klymchenko, A. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Accounts Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Irace, C.; Carallo, C.; Scavelli, F.; De Franceschi, M.S.; Esposito, T.; Gnasso, A. Blood Viscosity in Subjects With Normoglycemia and Prediabetes. Diabetes Care 2013, 37, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; King, M.R. Increased Protein Hydrophobicity in Response to Aging and Alzheimer’s disease. NIH Public Access 2014, 48, 1–23. [Google Scholar] [CrossRef]
- Estrada-Pérez, C.E.; Hassan, Y.A.; Tan, S. Experimental characterization of temperature sensitive dyes for laser induced fluorescence thermometry. Rev. Sci. Instrum. 2011, 82, 74901. [Google Scholar] [CrossRef]
- Cheng, B.; Bandi, V.; Yu, S.; D’Souza, F.; Nguyen, K.T.; Hong, Y.; Tang, L.; Yuan, B. The Mechanisms and Biomedical Applications of an NIR BODIPY-Based Switchable Fluorescent Probe. Int. J. Mol. Sci. 2017, 18, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® block-copolymers in medicine: from chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-F.; Tseng, H.-W.; Bahadur, P.; Chen, L.-J. Synergistic Effect of Binary Mixed-Pluronic Systems on Temperature Dependent Self-assembly Process and Drug Solubility. Polymers 2018, 10, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killoran, J.; McDonnell, S.O.; Gallagher, J.F.; O’Shea, D.F. A substituted BF2-chelated tetraarylazadipyrromethene as an intrinsic dual chemosensor in the 650–850 nm spectral range. New J. Chem. 2008, 32, 483–489. [Google Scholar] [CrossRef]
- Gresser, R.; Hummert, M.; Hartmann, H.; Leo, K.; Riede, M.K. Synthesis and Characterization of Near-Infrared Absorbing Benzannulated Aza-BODIPY Dyes. Chem. A Eur. J. 2011, 17, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Bandi, V.; El-Khouly, M.E.; Nesterov, V.N.; Karr, P.A.; Fukuzumi, S.; D’Souza, F. Self-Assembled via Metal-Ligand Coordination AzaBODIPY-Zinc Phthalocyanine and AzaBODIPY-Zinc Naphthalocyanine Conjugates: Synthesis, Structure, and Photoinduced Electron Transfer. J. Phys. Chem. C 2013, 117, 5638–5649. [Google Scholar] [CrossRef]
- Cheng, B.; Wei, M.; Liu, Y.; Pitta, H.; Xie, Z.; Hong, Y.; Nguyen, K.T.; Yuan, B. Development of Ultrasound-Switchable Fluorescence Imaging Contrast Agents Based on Thermosensitive Polymers and Nanoparticles. IEEE J. Sel. Top. Quantum Electron. 2013, 20, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Saremi, B.; Wei, M.-Y.; Liu, Y.; Cheng, B.; Yuan, B. Re-evaluation of biotin-streptavidin conjugation in Förster resonance energy transfer applications. J. Biomed. Opt. 2014, 19, 85008. [Google Scholar] [CrossRef] [Green Version]
- Haidekker, M.A.; Brady, T.; Lichlyter, D.; Theodorakis, E. Effects of solvent polarity and solvent viscosity on the fluorescent properties of molecular rotors and related probes. Bioorganic Chem. 2005, 33, 415–425. [Google Scholar] [CrossRef]
- Web Services at the European Bioinformatics Institute. Available online: https://www.ebi.ac.uk/chebi/ (accessed on 14 November 2019).
- Murov, S.L. Properties of Solvents Used in Organic Chemistry. Available online: http://murov.info/orgsolvents.htm (accessed on 14 November 2019).
- Kim, T.H.; Chen, Y.; Mount, C.; Gombotz, W.R.; Li, X.; Pun, S.H. Evaluation of Temperature-Sensitive, Indocyanine Green-Encapsulating Micelles for Noninvasive Near-Infrared Tumor Imaging. Pharm. Res. 2010, 27, 1900–1913. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Su, D.; Teoh, C.L.; Gao, N.; Xu, Q.-H.; Chang, Y.-T. A Simple BODIPY-Based Viscosity Probe for Imaging of Cellular Viscosity in Live Cells. Sensors 2016, 16, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidekker, M.A.; Theodorakis, E.A. Environment-sensitive behavior of fluorescent molecular rotors. J. Boil. Eng. 2010, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yu, H.; Xiao, Y. Replacing Phenyl Ring with Thiophene: An Approach to Longer Wavelength Aza-dipyrromethene Boron Difluoride (Aza-BODIPY) Dyes. J. Org. Chem. 2011, 77, 669–673. [Google Scholar] [CrossRef]
- Chen, J.; Reibenspies, J.; Derecskei-Kovacs, A.; Burgess, K. Through-space 13C–19F coupling can reveal conformations of modified BODIPY dyes†. Chem. Commun. 1999, 24, 2501–2502. [Google Scholar] [CrossRef]






| Fluorophore Name | Abbrev. Name | λex (nm) | λem (nm) | |
|---|---|---|---|---|
| 1 | Top Dimethyl amine ADPF2 | TOP DMAADP | 644 | 785/62 BP |
| 2 | Top Dimethyl amine ADPCNCA | TOP DMAADPCA | 644 | 808 LP |
| 3 | Benzannulated ADPF2 | Fused ADPF2 | 710 | 785/62 BP |
| 4 | ADP (OH)2 (Bottom) | ADP(OH)2(Bottom) | 655 | 711/25 BP |
| 5 | Top (OH)2 ADP | Top (OH)2 ADP | 655 | 711/25 BP |
| 6 | ADP-Di Sulphonic acid | ADP DI(SA) | 644 | 711/25 BP |
| Solvent | Solvent Type | ET(30) (kcal/mol) | Dielectric Constant ε |
|---|---|---|---|
| Water | Polar protic | 62.8 | 80.1 |
| DMSO | Dipolar aprotic | 45.1 | 46.7 |
| Dichloromethane | Polar aprotic | 40.7 | 9 |
| Benzene | Nonpolar | 34.3 | 2.3 |
| Toluene | Nonpolar | 33.9 | 2.4 |
| Glycerol | Polar protic | 57 | 42.5 |
| Ethylene glycol | Polar protic | 53.8 | 31.8 |
| Glycerol % | 0 | 16 | 50 | 75 | 92 | 100 |
| Viscosity (m Pa.s) | 12.7 | 20.8 | 53.5 | 117.0 | 273.5 | 634.5 |
| Pluronic Nanoparticles | ION-to-IOFF Edge Method | ION-to-IOFF Vicinity Method | LCST * (°C) Nanoparticles | TBW (°C) | Lifetime (ns) (OFF to ON Edge Method) |
|---|---|---|---|---|---|
| F-127_#1 | 8.37 ± 0.11 | 13.74 ± 1.39 | 25 | 27 | 0.41 ± 0.01 to 1.09 ± 0.01 |
| F-127_#2 | 4.97 ± 0.4 | 6.36 ± 0.17 | 25 | 6 | 0.27 ± 0.1 to 0.75 ± 0.06 |
| F-127_#3 | 5.40 ± 0.76 | 5.75 ± 0.34 | 19 | 9 | 1.42 ± 0.45 to 1.81 ± 0.05 |
| F-127_#4 | 46.74 ± 3.35 | 109.1 ± 18.9 | 25 | 6 | 2.0 ± 0.14 to 3.02 ± 0.03 |
| F-127_#5 | 3.59 ± 0.08 | 4.65 ± 0.14 | 25 | 6 | 2.55 ± 0.07 to 3.02 ± 0.17 |
| F-127_#6 | 1.81 ± 0.15 | 2.04 ± 0.18 | 13 | 15 | 2.96 ± 0.23 to 3.23 ± 0.08 |
| F-98_#4 | 62.26 ± 9.9 | 137.23 ± 0.15 | 34 | 6 | 1.5 ± 0.1 to 1.65 ± 0.05 |
| F-68_#4 | 72.74 ± 0.81 | 102.07 ± 3.72 | 61 | 9 | 1.14 0.3 to 2.33 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saremi, B.; Bandi, V.; Kazemi, S.; Hong, Y.; D’Souza, F.; Yuan, B. Exploring NIR Aza-BODIPY-Based Polarity Sensitive Probes with ON-and-OFF Fluorescence Switching in Pluronic Nanoparticles. Polymers 2020, 12, 540. https://doi.org/10.3390/polym12030540
Saremi B, Bandi V, Kazemi S, Hong Y, D’Souza F, Yuan B. Exploring NIR Aza-BODIPY-Based Polarity Sensitive Probes with ON-and-OFF Fluorescence Switching in Pluronic Nanoparticles. Polymers. 2020; 12(3):540. https://doi.org/10.3390/polym12030540
Chicago/Turabian StyleSaremi, Bahar, Venugopal Bandi, Shahrzad Kazemi, Yi Hong, Francis D’Souza, and Baohong Yuan. 2020. "Exploring NIR Aza-BODIPY-Based Polarity Sensitive Probes with ON-and-OFF Fluorescence Switching in Pluronic Nanoparticles" Polymers 12, no. 3: 540. https://doi.org/10.3390/polym12030540
APA StyleSaremi, B., Bandi, V., Kazemi, S., Hong, Y., D’Souza, F., & Yuan, B. (2020). Exploring NIR Aza-BODIPY-Based Polarity Sensitive Probes with ON-and-OFF Fluorescence Switching in Pluronic Nanoparticles. Polymers, 12(3), 540. https://doi.org/10.3390/polym12030540