Thermosensitive Hydrogel for Encapsulation and Controlled Release of Biocontrol Agents to Prevent Peanut Aflatoxin Contamination
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
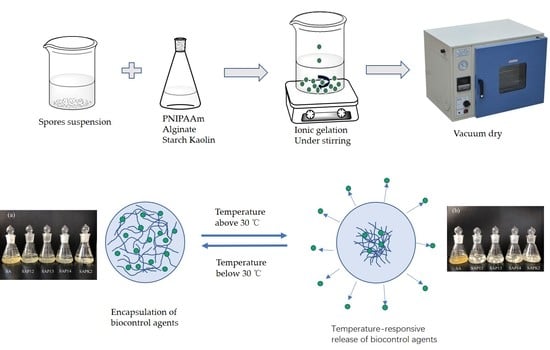
2.2. Preparation of Thermosensitive Hydrogel Beads
2.2.1. Preparation of Spore Suspensions
2.2.2. Synthesis of PNIPAAm
2.2.3. Preparation of Thermosensitive Hydrogel Beads
2.3. Characterization
2.4. Determination of Thermosensitive Hydrogel Temperature-Sensitive Behavior and LCST
2.5. Swelling Studies
2.6. Rheological Properties
2.7. Release Measurements
2.7.1. Entrapment Efficiency of Spores
2.7.2. Release Properties of Spores
2.7.3. Release Kinetics of Spores
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Formulation Parameters on the Characteristics of the Beads
3.2. Thermosensitive Behavior of Semi-IPN Hydrogels
3.3. Characterization
3.3.1. FTIR
3.3.2. Thermogravimetric Analysis
3.3.3. SEM
3.3.4. Swelling Studies
3.4. Rheological Properties
3.5. Release Properties of Spores from Thermosensitive Hydrogel Beads
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alaniz Zanon, M.S.; Chiotta, M.L.; Giaj-Merlera, G.; Barros, G.; Chulze, S. Evaluation of potential biocontrol agent for aflatoxin in Argentinean peanuts. Int. J. Food Microbiol. 2013, 162, 220–225. [Google Scholar] [CrossRef]
- Horn, B.W.; Dorner, J.W. Effect of nontoxigenic Aspergillus flavus and A. parasiticus on aflatoxin contamination of wounded peanut seeds inoculated with agricultural soil containing natural fungal populations. Biocontrol Sci. Technol. 2009, 19, 249–262. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Mycotoxins in Australia: Biocontrol of aflatoxin in peanuts. Mycopathologia 2006, 162, 233–243. [Google Scholar] [CrossRef]
- Dorner, J. Development of biocontrol technology to manage aflatoxin contamination in peanuts. Peanut Sci. 2009, 36, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Accinelli, C.; Abbas, H.K. New perspectives for the application of bioplastic materials in the biocontrol ofAspergillus flavusin corn. Toxin Rev. 2011, 30, 71–78. [Google Scholar] [CrossRef]
- Accinelli, C.; Sacca, M.L.; Abbas, H.K.; Zablotowicz, R.M.; Wilkinson, J.R. Use of a granular bioplastic formulation for carrying conidia of a non-aflatoxigenic strain of Aspergillus flavus. Bioresour. Technol. 2009, 100, 3997–4004. [Google Scholar] [CrossRef] [PubMed]
- Accinelli, C.; Abbas, H.K.; Vicari, A.; Shier, W.T. Evaluation of recycled bioplastic pellets and a sprayable formulation for application of an Aspergillus flavus biocontrol strain. Crop Prot. 2015, 72, 9–15. [Google Scholar] [CrossRef]
- Azwa, Z.N.; Yousif, B.F.; Manalo, A.C.; Karunasena, W. A review on the degradability of polymeric composites based on natural fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef] [Green Version]
- Domian, E.; Brynda-Kopytowska, A.; Cenkier, J.; Świrydow, E. Selected properties of microencapsulated oil powders with commercial preparations of maize OSA starch and trehalose. J. Food Eng. 2015, 152, 72–84. [Google Scholar] [CrossRef]
- Rong, L.; Shoemaker, C.F.; Xiaoqing, Y.; Fang, Z.; Qingrong, H. Stability and bioaccessibility of β-carotene in nanoemulsions stabilized by modified starches. J. Agric. Food Chem. 2013, 61, 1249–1257. [Google Scholar]
- Wang, S.; Chen, X.; Shi, M.; Zhao, L.; Wei, L.; Chen, Y.; Lu, M.; Wu, J.; Yuan, Q.; Yuan, L. Absorption of whey protein isolated (WPI)-stabilized β-Carotene emulsions by oppositely charged oxidized starch microgels. Food Res. Int. 2015, 67, 315–322. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Gao, Q.; Liu, X.; Tong, Z.J.C.P. Alginate–calcium carbonate porous microparticle hybrid hydrogels with versatile drug loading capabilities and variable mechanical strengths. Carbohydr. Polym. 2008, 71, 476–480. [Google Scholar] [CrossRef]
- Kruif, C.G.D.; Weinbreck, F.; Vries, R.D. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2005, 9, 340–349. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Deladino, L.; Martino, M. Corn starch-calcium alginate matrices for the simultaneous carrying of zinc and yerba mate antioxidants. LWT-Food Sci. Technol. 2014, 59, 641–648. [Google Scholar]
- Locatelli, G.O.; Santos, G.F.D.; Botelho, P.S.; Finkler, C.L.L.; Bueno, L.A. Development of Trichoderma sp. formulations in encapsulated granules (CG) and evaluation of conidia shelf-life. Biol. Control 2018, 117, 21–29. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Gupta, A. A study towards release dynamics of thiram fungicide from starch-alginate beads to control environmental and health hazards. J. Hazard. Mater. 2009, 161, 208–216. [Google Scholar] [CrossRef]
- Fernandezperez, M. Controlled release systems to prevent the agro-environmental pollution derived from pesticide use. J. Environ. Sci. Health Part B 2007, 42, 857–862. [Google Scholar] [CrossRef]
- Feng, J.; Dou, J.; Wu, Z.; Yin, D.; Wu, W. Controlled Release of Biological Control Agents for Preventing Aflatoxin Contamination from Starch(-)Alginate Beads. Molecules 2019, 24, 1858. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Liu, J.; Cao, S.; Tan, H.; Li, J.; Xu, F.; Zhang, X. Drug release behaviors of a pH sensitive semi-interpenetrating polymer network hydrogel composed of poly(vinyl alcohol) and star poly[2 -(dimethylamino)ethyl methacrylate]. Int. J. Pharm. 2011, 416, 104–109. [Google Scholar] [CrossRef]
- Lo, C.; Zhu, D.; Jiang, H. An infrared-light responsive graphene-oxide incorporated poly(N-isopropylacrylamide) hydrogel nanocomposite. Soft Matter 2011, 7, 5604–5609. [Google Scholar] [CrossRef]
- Sun, X.; Shi, J.; Xu, X.; Cao, S. Chitosan coated alginate/poly(N-isopropylacrylamide) beads for dual responsive drug delivery. Int. J. Biol. Macromol. 2013, 59, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.J.C.P. Xylan-based temperature/pH sensitive hydrogels for drug controlled release. Carbohydr. Polym. 2016, 151, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Chen, L.; Zhang, R.; Guo, G. Cell growth and desorption on the surface of temperaturesensitive semi-IPNs hydrogels based on silk sericin. J. Wuhan Univ. Technol. -Mater. Sci. Ed. 2012, 27, 907–910. [Google Scholar] [CrossRef]
- Basri, M.; Harun, A.; Ahmad, M.B.; Razak, C.N.A.; Salleh, A.B. Immobilization of lipase on poly(N-vinyl-2-pyrrolidone-co-styrene) hydrogel. J. Appl. Polym. Sci. 2010, 82, 1404–1409. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Jing, M.; Liu, S.; Feng, J.; Wu, H.; Zhou, Y.; Zhang, X.; Ma, Z. Self-assembled mixed micelle loaded with natural pyrethrins as an intelligent nano-insecticide with a novel temperature-responsive release mode. Chem. Eng. J. 2019, 361, 1381–1391. [Google Scholar] [CrossRef]
- Xu, X.; Bai, B.; Wang, H.; Suo, Y. A Near-Infrared and Temperature-Responsive Pesticide Release Platform through Core-Shell Polydopamine@PNIPAm Nanocomposites. Acs Appl. Mater. Interfaces 2017, 9, 6424–6432. [Google Scholar] [CrossRef]
- Heitfeld, K.A.; Guo, T.; Yang, G.; Schaefer, D.W. Temperature responsive hydroxypropyl cellulose for encapsulation. Mater. Sci. Eng. C 2008, 28, 374–379. [Google Scholar] [CrossRef]
- De Moura, M.R.; Ahmad Aouada, F.; Favaro, S.L.; Radovanovic, E.; Forti Rubira, A.; Muniz, E.C. Release of BSA from porous matrices constituted of alginate–Ca2+ and PNIPAAm-interpenetrated networks. Mater. Sci. Eng. C 2009, 29, 2319–2325. [Google Scholar] [CrossRef]
- Liang, L.; Liu, J.; Gong, X. Thermosensitive Poly(N-isopropylacrylamide)−Clay Nanocomposites with Enhanced Temperature Response. Langmuir 2000, 16, 9895–9899. [Google Scholar] [CrossRef]
- Gao, Q.Z.; Liu, S.Z.; Mei, H.Z.; Jing, H.M.; Bo, R.L. Preparation and characterization of pH- and temperature-responsive semi–interpenetrating polymer network hydrogels based on linear sodium alginate and crosslinked poly(N-isopropylacrylamide). J. Appl. Polym. Sci. 2010, 97, 1931–1940. [Google Scholar]
- Leal, D.; De Borggraeve, W.; Encinas, M.V.; Matsuhiro, B.; Muller, R. Preparation and characterization of hydrogels based on homopolymeric fractions of sodium alginate and PNIPAAm. Carbohydr. Polym. 2013, 92, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, R.P.; Mitchell, G.R.; Vasile, C. Rheological and thermal behaviour of poly(N-isopropylacrylamide)/alginate smart polymeric networks. Polym. Int. 2011, 60, 1398–1407. [Google Scholar] [CrossRef]
- Shi, J.; Alves, N.M.; Mano, J.F. Drug release of pH/temperature-responsive calcium alginate/poly(N-isopropylacrylamide) semi-IPN beads. Macromol. Biosci. 2006, 6, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piai, J.F.; De Moura, M.R.; Rubira, A.F.; Muniz, E.C. Kinetic Study of Bovine Serum Albumin (BSA) Released from Alginate-Ca2+/PNIPAAm Hydrogels. Macromol. Symp. 2010, 266, 108–113. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Shi, J.; Liu, L.; Sun, X.; Cao, S.; Mano, J.F. Biomineralized polysaccharide beads for dual-stimuli-responsive drug delivery. Macromol. Biosci. 2008, 8, 260–267. [Google Scholar] [CrossRef]
- Cui, Y.; Xing, Z.; Yan, J.; Lu, Y.; Xiong, X.; Zheng, L. Thermosensitive Behavior and Super-Antibacterial Properties of Cotton Fabrics Modified with a Sercin-NIPAAm-AgNPs Interpenetrating Polymer Network Hydrogel. Polym. (Basel) 2018, 10, 818. [Google Scholar] [CrossRef] [Green Version]
- Alaniz Zanon, M.S.; Barros, G.G.; Chulze, S.N. Non-aflatoxigenic Aspergillus flavus as potential biocontrol agents to reduce aflatoxin contamination in peanuts harvested in Northern Argentina. Int. J. Food Microbiol. 2016, 231, 63–68. [Google Scholar] [CrossRef]
- Accinelli, C.; Mencarelli, M.; Saccà, M.L.; Vicari, A.; Abbas, H.K. Managing and monitoring of Aspergillus flavus in corn using bioplastic-based formulations. Crop Prot. 2012, 32, 30–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Mangond, B.S.; Mutalik, S.; Sa, B. Interpenetrating polymer network microcapsules of gellan gum and egg albumin entrapped with diltiazem–resin complex for controlled release application. Carbohydr. Polym. 2011, 83, 1001–1007. [Google Scholar] [CrossRef]
- Ueoka, H.; Shimomura, O.; Ueda, K.; Inada, K.; Nomura, R. Release behavior of a polyanion-crosslinked chitosan-poly(N-isopropylacrylamide) gel thermoresponsive material. J. Appl. Polym. Sci. 2018, 135, 41. [Google Scholar] [CrossRef]
- Puttipipatkhachorn, S.; Pongjanyakul, T.; Priprem, A. Molecular interaction in alginate beads reinforced with sodium starch glycolate or magnesium aluminum silicate, and their physical characteristics. Int. J. Pharm. 2005, 293, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.K.; Dusseault, J.; Polizu, S.; Ménard, M.; Hallé, J.P.; Yahia, L.J.B. Physicochemical model of alginate-poly-L-lysine microcapsules defined at the micrometric/nanometric scale using ATR-FTIR, XPS, and ToF-SIMS. Biomaterials 2005, 26, 6950–6961. [Google Scholar] [CrossRef] [PubMed]
- Mohan, Y.M.; Premkumar, T.; Joseph, D.K.; Geckeler, K.E.J.R.; Polymers, F. Stimuli-responsive poly(N -isopropylacrylamide-co-sodium acrylate) hydrogels: A swelling study in surfactant and polymer solutions. React. Funct. Polym. 2007, 67, 844–858. [Google Scholar] [CrossRef]
- Sakugawa, K.; Ikeda, A.; Takemura, A.; Ono, H. Simplified method for estimation of composition of alginates by FTIR. J. Appl. Polym. Sci. 2004, 93, 1372–1377. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Kumar, R.; Gupta, A. Controlled release of the fungicide thiram from starch–alginate–clay based formulation. Appl. Clay Sci. 2009, 45, 76–82. [Google Scholar] [CrossRef]
- Wang, Y.; Chao, L.; Peng, L.; Ahmed, Z.; Ping, X.; Bai, X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydr. Polym. 2010, 82, 895–903. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Kumar, R.; Gupta, A. Controlled release of thiram from neem-alginate-clay based delivery systems to manage environmental and health hazards. Appl. Clay Sci. 2010, 47, 384–391. [Google Scholar] [CrossRef]
- Adzmi, F.; Meon, S.; Musa, M.H.; Yusuf, N.A. Preparation, characterisation and viability of encapsulated Trichoderma harzianum UPM40 in alginate-montmorillonite clay. J. Microencapsul. 2012, 29, 205–210. [Google Scholar] [CrossRef]
- Yang, L.; Shi, J.; Zhou, X.; Cao, S. Hierarchically organization of biomineralized alginate beads for dual stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2015, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F. Stimuli-Responsive Polymeric Systems for Biomedical Applications. Adv. Eng. Mater. 2010, 10, 515–527. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Wang, A. Utilization of starch and clay for the preparation of superabsorbent composite. Bioresour. Technol. 2007, 98, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Ghasemzadeh, H.; Soleyman, R. Synthesis, characterization, and swelling behavior of alginate-g-poly(sodium acrylate)/kaolin superabsorbent hydrogel composites. J. Appl. Polym. Sci. 2007, 105, 2631–2639. [Google Scholar] [CrossRef]
- Ju, H.K.; Kim, S.Y.; Lee, Y.M. pH/temperature-responsive behaviors of semi-IPN and comb-type graft hydrogels composed of alginate and poly(N-isopropylacrylamide). Polymer 2001, 42, 6851–6857. [Google Scholar] [CrossRef]
- Cataldo, S.; Gianguzza, A.; Merli, M.; Muratore, N.; Piazzese, D.; Liveri, M.L. Experimental and robust modeling approach for lead(II) uptake by alginate gel beads: Influence of the ionic strength and medium composition. J. Colloid Interface Sci. 2014, 434, 77–88. [Google Scholar] [CrossRef]
- Soledad Lencina, M.M.; Iatridi, Z.; Villar, M.A.; Tsitsilianis, C. Thermoresponsive hydrogels from alginate-based graft copolymers. Eur. Polym. J. 2014, 61, 33–44. [Google Scholar] [CrossRef]
- Liu, R.; Fraylich, M.; Saunders, B.R. Thermoresponsive copolymers: From fundamental studies to applications. Colloid Polym. Sci. 2009, 287, 627–643. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, C.; Fan, L.; Wang, L.; Zheng, H. Preparation of dual crosslinked alginate–chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int. J. Pharm. 2007, 336, 329–337. [Google Scholar] [CrossRef]
- Shi, J.; Alves, N.M.; Mano, J.F. Chitosan coated alginate beads containing poly(N-isopropylacrylamide) for dual-stimuli-responsive drug release. J. Biomed. Mater. Res. Part B 2008, 84, 595–603. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wu, Z.; Tu, L.; Han, Y.; Zhang, G.; Li, C. Encapsulation and characterization of slow-release microbial fertilizer from the composites of bentonite and alginate. Appl. Clay Sci. 2015, 109–110, 68–75. [Google Scholar] [CrossRef]
- Li, B.; Jiang, Y.; Liu, Y.; Wu, Y.; Yu, H.; Zhu, M. Novel poly(N-isopropylacrylamide)/clay/poly(acrylamide) IPN hydrogels with the response rate and drug release controlled by clay content. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 96–106. [Google Scholar] [CrossRef]














| Sample | PNIPAAm/Alginate | CaCl2 Solution Concentration | Starch | Alginate | Kaolin |
|---|---|---|---|---|---|
| (v/v) | (% w/v) | (% w/v) | (% w/v) | (% w/v) | |
| SA | / | 2 | 10 | 1.5 | / |
| SAP12 | 1:2 | 2 | 10 | 1.5 | / |
| SAP13 | 1:3 | 2 | 10 | 1.5 | / |
| SAP14 | 1:4 | 2 | 10 | 1.5 | / |
| SAPK1 | 1:3 | 2 | 10 | 1.5 | 1 |
| SAPK2 | 1:3 | 2 | 10 | 1.5 | 2 |
| SAPK3 | 1:3 | 2 | 10 | 1.5 | 3 |
| Sample | Beads Weight (g) | Average Bead Diameter (mm) | Encapsulation Efficiency (%) | LCST (°C) |
|---|---|---|---|---|
| SA | 2.13 | 1.93 ± 0.08 c | 48.25 ± 3.67 d | / |
| SAP12 | 2.81 | 2.55 ± 0.13 a,b,* | 67.44 ± 4.36 c | 29.2 |
| SAP13 | 2.38 | 2.41 ± 0.05 a,b | 66.65 ± 2.94 c | 29.3 |
| SAP14 | 2.49 | 2.33 ± 0.18 b | 43.25 ± 1.88 e | 30.0 |
| SAPK1 | 2.68 | 2.46 ± 0.16 a,b | 64.36 ± 2.16 c | 29.7 |
| SAPK2 | 2.95 | 2.54 ± 0.21 a,b | 90.48 ± 1.53 a | 29.5 |
| SAPK3 | 3.11 | 2.63 ± 0.12 a | 75.32 ± 2.22 b | 29.3 |
| Sample | Swelling Ratio | ||
|---|---|---|---|
| 25 °C | 30 °C | 35 °C | |
| SAP12 | 29.03 ± 2.13 b | 22.08 ± 0.69 c | 21.24 ± 0.56 b,c |
| SAP13 | 28.47 ± 3.02 b | 23.25 ± 1.53 b,c | 22.89 ± 1.34 b |
| SAP14 | 29.04 ± 1.85 b | 24.22 ± 2.22 b | 21.33 ± 1.54 b,c |
| SAPK1 | 26.23 ± 0.55 b,c | 22.46 ± 1.12 b,c | 21.75 ± 0.83 b,c |
| SAPK2 | 23.76 ± 1.99 c | 21.32 ± 0.95 b,c | 20.07 ± 0.97 c,d |
| SAPK3 | 22.54 ± 1.21 c | 20.94 ± 0.95 c | 18.87 ± 0.67 d |
| SA | 42.64 ± 2.65 a | 49.93 ± 1.38 a | 52.90 ± 2.02 a |
| Temperature (℃) | Formulation | n | K | Mechanism |
|---|---|---|---|---|
| 25 | SAP12 | 0.36 | 0.15 | Normal Fickian |
| SAP13 | 0.35 | 0.15 | Normal Fickian | |
| SAP14 | 0.39 | 0.13 | Normal Fickian | |
| SAPK1 | 0.45 | 0.08 | Normal Fickian | |
| SAPK2 | 0.49 | 0.06 | Normal Fickian | |
| SAPK3 | 0.48 | 0.09 | Normal Fickian | |
| SA | 0.42 | 0.11 | Normal Fickian | |
| 30 | SAP12 | 0.09 | 0.55 | Normal Fickian |
| SAP13 | 0.09 | 0.53 | Normal Fickian | |
| SAP14 | 0.08 | 0.56 | Normal Fickian | |
| SAPK1 | 0.11 | 0.51 | Normal Fickian | |
| SAPK2 | 0.10 | 0.49 | Normal Fickian | |
| SAPK3 | 0.09 | 0.46 | Normal Fickian | |
| SA | 0.24 | 0.26 | Normal Fickian | |
| 35 | SAP12 | 0.06 | 0.67 | Normal Fickian |
| SAP13 | 0.07 | 0.64 | Normal Fickian | |
| SAP14 | 0.06 | 0.67 | Normal Fickian | |
| SAPK1 | 0.06 | 0.58 | Normal Fickian | |
| SAPK2 | 0.05 | 0.62 | Normal Fickian | |
| SAPK3 | 0.08 | 0.60 | Normal Fickian | |
| SA | 0.18 | 0.35 | Normal Fickian |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Dou, J.; Zhang, Y.; Wu, Z.; Yin, D.; Wu, W. Thermosensitive Hydrogel for Encapsulation and Controlled Release of Biocontrol Agents to Prevent Peanut Aflatoxin Contamination. Polymers 2020, 12, 547. https://doi.org/10.3390/polym12030547
Feng J, Dou J, Zhang Y, Wu Z, Yin D, Wu W. Thermosensitive Hydrogel for Encapsulation and Controlled Release of Biocontrol Agents to Prevent Peanut Aflatoxin Contamination. Polymers. 2020; 12(3):547. https://doi.org/10.3390/polym12030547
Chicago/Turabian StyleFeng, Jiachang, Jianpeng Dou, Youzhen Zhang, Zidan Wu, Dongxue Yin, and Wenfu Wu. 2020. "Thermosensitive Hydrogel for Encapsulation and Controlled Release of Biocontrol Agents to Prevent Peanut Aflatoxin Contamination" Polymers 12, no. 3: 547. https://doi.org/10.3390/polym12030547
APA StyleFeng, J., Dou, J., Zhang, Y., Wu, Z., Yin, D., & Wu, W. (2020). Thermosensitive Hydrogel for Encapsulation and Controlled Release of Biocontrol Agents to Prevent Peanut Aflatoxin Contamination. Polymers, 12(3), 547. https://doi.org/10.3390/polym12030547