Surface Studies of UV Irradiated Polypropylene Films Modified with Mineral Fillers Designed as Piezoelectric Materials
Abstract
:1. Introduction
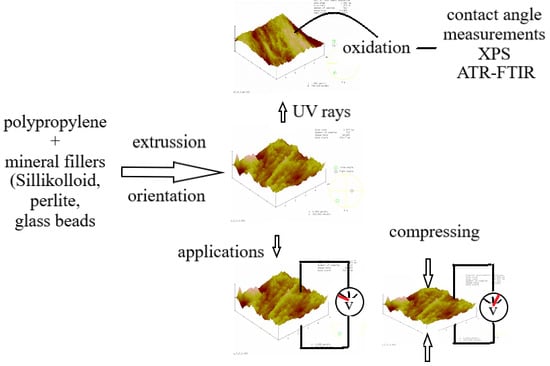
2. Materials and Methods
2.1. Materials
2.2. Composite Extrusion
2.3. Weathering Conditions
2.4. Contact Angle Measurements
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.7. Atomic Force Microscopy (AFM)
3. Results and Discussion
3.1. Contact Angle Measurements
3.2. Surface Composition Analysis
3.3. ATR-FTIR Spectroscopy Results
3.4. AFM Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramadan, K.S.; Sameoto, D.; Evoy, S. A review of piezoelectric polymers as functional materials for electromechanical transducers. Smart Mater. Struct. 2014, 23, 033001. [Google Scholar] [CrossRef]
- Sappati, K.K.; Bhadra, S. Piezoelectric Polymer and Paper Substrates: A Review. Sensors 2018, 18, 3605. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Unnikrishnan, L.; Kumar Nayak, S.; Mohanty, S. Advances in Piezoelectric Polymer Composites for Energy Harvesting Applications: A Systematic Review. Macromol. Mater. Eng. 2019, 304, 1800463. [Google Scholar] [CrossRef] [Green Version]
- Mohebbi, A.; Mighri, F.; Ajji, A.; Rodrigue, D. Cellular Polymer Ferroelectret: A Review on Their Development and Their Piezoelectric Properties. Adv. Polym. Technol. 2018, 37, 468–483. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Królikowski, B.; Klimiec, E.; Kowalonek, J. New piezoelectric composites based on isotactic polypropylene filled with silicate. J. Mater. Sci. Mater. Electron. 2017, 28, 6435–6447. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, H.; Chylińska, M.; Klimiec, E.; Królikowski, B.; Sionkowski, G.; Machnik, M. Piezo-electrets from polypropylene composites doped with mineral fillers. Pure Appl. Chem. 2019, 91, 967–982. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Królikowski, B.; Klimiec, E.; Bajer, D. The structure of the materials intended for piezoelectric composites based on polypropylene. Polimery 2017, 62, 539–547. [Google Scholar] [CrossRef]
- Rabek, J.F. Polymer Photodegradation. Mechanism and Experimental Methods; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Carlsson, D.J.; Wiles, D.M. The Photodegradation of Polypropylene Films. Photolysis of Ketonic Oxidation Products. Macromolecules 1969, 2, 587–597. [Google Scholar] [CrossRef]
- Tidjani, A. Photooxidation of Polypropylene Under Natural and Accelerated Conditions. J. Appl. Polym. Sci. 1997, 64, 2497–2503. [Google Scholar] [CrossRef]
- Gardette, J.-L.; Sinturel, C.H.; Lemaire, J. Photooxidation of fire retarded polypropylene. Polym. Degrad. Stab. 1999, 64, 411–417. [Google Scholar] [CrossRef]
- Sinturel, C.; Lemaire, J.; Gardette, J.-L. Photooxidation of fire retarded polypropylene II. Photooxidation mechanism. Eur. Polym. J. 1999, 35, 1783–1790. [Google Scholar] [CrossRef]
- Halim Hamid, S. Handbook of Polymer Degradation. Second Edition, Revised and Expanded; Marcel Decker Inc.: New York, NY, USA, 2000. [Google Scholar]
- Philippart, J.-L.; Posada, F.; Gardette, J.-L. Mass spectroscopy analysis of volatile photoproducts in photooxidation of polypropylene. Polym. Degrad. Stab. 1995, 49, 285–290. [Google Scholar] [CrossRef]
- Bertoldo, M.; Bronco, S.; Cappelli, C.; Gragnoli, T.; Andreotti, L. Combining Theory and Experiment to Study the Photooxidation of Polyethylene and Polypropylene. J. Phys. Chem. B 2003, 107, 11880–11888. [Google Scholar] [CrossRef]
- SILLIKOLLOID P 87—Hoffmann Mineral—Functional Fillers. Available online: https://www.hoffmann-mineral.com/Products/Sillitin-Sillikolloid/SILLIKOLLOID-P-87 (accessed on 2 December 2019).
- Frąszczak, Z.; Królikowski, B.; Buchelt, A. Modification of polymeric materials with perlite. Part II. Przem. Chem. 2014, 93, 1000–1003. [Google Scholar]
- Frąszczak, Z.; Królikowski, B.; Buchelt, A. Perlite as a filler for thermoplastics. Przetwórstwo Tworzyw 2014, 3, 199–202. [Google Scholar]
- MinTron 7™ Product Data Sheet—RockTron—Yumpu. Available online: https://www.yumpu.com/en/document/view/7180532/mintron-7tm-product-data-sheet-rocktron (accessed on 2 December 2019).
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Mattausch, H.; Laske, S.; Cirar, K.; Flachberger, H.; Holzer, C. Influence of Processing Conditions on The Morphology of Expanded Perlite/Polypropylene Composites. AIP Conf. Proc. 2014, 1593, 482–486. [Google Scholar]
- Zhao, J.; Liu, X.; Yang, R.; Yu, J. Disappearance of the filler—An interesting interfacial evolution during the photooxidative aging of polypropylene composites. J. Appl. Polym. Sci. 2015, 132, 42546. [Google Scholar] [CrossRef]
- Akin-Öktem, G.; Tanrisinibilir, S.; Tinçer, T. Study on Mechanical Properties of Perlite-Filled Gamma-Irradiated Polypropylene. J. Appl. Polym. Sci. 2001, 81, 2670–2678. [Google Scholar] [CrossRef]
- Pawlak, A. Cavitation During Deformation of Polymers on the Example of Polypropylene. J. Appl. Polym. Sci. 2012, 125, 4177–4187. [Google Scholar] [CrossRef]
- Abbasi Mahmoodabadi, H.; Haghighat Kish, M.; Asianzadeh, S. Photodegradation of partially oriented and drawn polypropylene filaments. J. Appl. Polym. Sci. 2018, 135, 45716. [Google Scholar] [CrossRef]
- Suljovrujic, E. The influence of molecular orientation on the crosslinking/oxidative behaviour of iPP exposed to gamma radiation. Eur. Polym. J. 2009, 45, 2068–2078. [Google Scholar] [CrossRef]
- Li, J.; Yang, R.; Yu, J.; Liu, Y. Natural photo-aging degradation of polypropylene nanocomposites. Polym. Degrad. Stab. 2008, 93, 84–89. [Google Scholar] [CrossRef]
- Chernomordik, B.D.; Russell, H.B.; Cvelbar, U.; Jasinski, J.B.; Kumar, V.; Dautsch, T.; Sunkara, M.K. Photoelectrochemical activity of as-grown, α-Fe2O3 nanowire array electrodes for water splitting. Nanotechnology 2012, 23, 194009. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhaoxia, J.; Tian, X.; Huan, M.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.-P.; et al. Use of Metal Oxide Nanoparticle Band Gap to Develop a Predictive Paradigm for Oxidative Stress and Acut Pulmonary Inflammation. Am. Chem. Soc. Nano 2012, 6, 4349–4368. [Google Scholar]
- Qi, L.; Ding, Y.; Dong, Q.; Wen, B.; Wang, F.; Zhang, S.; Yang, M. Photostabilization of Polypropylene by Surface Modified Rutile-Type TiO2 Nanorods. J. Appl. Polym. Sci. 2014, 131, 40601. [Google Scholar] [CrossRef]
- Liauw, C.M.; Childs, A.; Allen, N.S.; Edge, M.; Franklin, K.R.; Collopy, D.G. Effect of interactions between stabilizeres and silica used for anti-bloking applications on UV and thermal stability of polyolefin. 2. Degradation studies. Polym. Degrad. Stab. 1999, 65, 207–215. [Google Scholar] [CrossRef]
- Mailhot, B.; Morlat, S.; Gardette, J.-L.; Boucard, S.; Duchet, J.; Gérard, J.-F. Photodegradation of polypropylene nanocomposites. Polym. Degrad. Stab. 2003, 82, 163–167. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Garton, A.; Wiles, D.M. The Weatherability of Polypropylene Monofilaments. Effects of Fiber Production Conditions. J. Appl. Polym. Sci. 1977, 21, 2963–2978. [Google Scholar] [CrossRef]
- Garton, A.; Carlsson, D.J.; Sturgeon, P.Z.; Wiles, D.M. The The Photo-oxidation of Polypropylene Monofilaments. Part III: Effects of Filament Morphology. Text. Res. J. 1977, 47, 423–428. [Google Scholar] [CrossRef]
- Navaneetha Pandiyaraj, K.; Ram Kumar, M.C.; Arun Kumar, A.; Padmanabhan, P.V.A.; Deshmukh, R.R.; Bah, M.; Ismat Shah, S.; Su, P.-G.; Halleluyah, M., Jr.; Halim, A.S. Tailoring the surface properties of polypropylene films through cold atmospheric pressure plasma (CAPP) assisted polymerization and immobilization of biomolecules for enhancement of anti-coagulation activity. Appl. Surf. Sci. 2016, 370, 545–556. [Google Scholar] [CrossRef]
- Nikitin, D.; Choukourov, A.; Titov, V.; Kuzmiecheva, L.; Lipatova, I.; Mezina, E.; Aleksandriiskii, V.; Shelemin, A.; Khalakan, I.; Slavinska, D.; et al. In situ coupling of chitosan onto polypropylene foils by an Atmospheric Plasma Air Glow Discharge with a liquid cathode. Carbohydr. Polym. 2016, 154, 30–39. [Google Scholar] [CrossRef]
- O’Connell, C.; Sherlock, R.; Ball, M.D.; Aszalós-Kiss, B.; Prendergast, U.; Glynn, T.J. Investigation of the hydrophobic recovery of various polymeric biomaterials after 172 nm UV treatment using contact angle, surface free energy and XPS measurement. Appl. Surf. Sci. 2009, 255, 4405–4413. [Google Scholar] [CrossRef]
- Encinas, N.; Díaz-Berniito, B.; Abenojar, J.; Martínez, M.A. Extreme durability of wettability changes on polyolefin surfaces by atmospheric pressure plasma torch. Surf. Coat. Technol. 2010, 205, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Żenkiewicz, M. Adhesion and Modification of Outer Layer of High-Molecular Plastics; WNT: Warsaw, Poland, 2000. [Google Scholar]
- Chen, H.; Shi, X.; Zhu, Y.; Xu, J. Enrichment of Poly (butyl methacrylate) and Its Graft Copolymer of Polybutadiene on the Surface of Polypropylene blend. J. Appl. Polym. Sci. 2008, 107, 3049–3057. [Google Scholar] [CrossRef]
- Zieliński, W. Spectroscopic Methods and Their Applications for Identification of Organic Compounds; WNT: Warsaw, Poland, 1995. [Google Scholar]
- Ranjbar, N.; Mehrali, M.; Behnia, A.; Pordsari, A.J.; Mehrali, M.; Alengaram, U.J.; Jumaat, M.Z. A Comprehensive Study of the Polypropylene Fiber Reinforced Fly Ash Based Geopolymer. PLoS ONE 2016, 11, e0147546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless Melt-Electrospinning of Polypropylene Nanofibres. J. Nanomater. 2012, 2012, 382639. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, T.; Zhang, A. Generation Mechanism of Oxidation Products during the Air Atmosphere Oxidation of SEBS/PP Blends: Tracked by 2D Correlation Infrared Spectroscopy. Macromol. Chem. Phys. 2016, 217, 2501–2516. [Google Scholar] [CrossRef]
- Rjeb, A.; Tajounte, L.; Chafik El Idrissi, M.; Letarte, S.; Adnot, A.; Roy, D.; Claire, Y.; Périchaund, A.; Kaloustian, J. IR Spectroscopy Study of Polypropylene Natural Aging. J. Appl. Polym. Sci. 2000, 77, 1742–1748. [Google Scholar] [CrossRef]
- Kalapat, N.; Amornsakchai, T. Surface modification of biaxially oriented (BOPP) film using acrylic acid-corona treatment: Part I. Properties and characterization of treated films. Surf. Coat. Technol. 2012, 207, 594–601. [Google Scholar] [CrossRef]








| Filler | Producer | Chemical Analysis | Bulk Density | Particle Size | Particle Shape |
|---|---|---|---|---|---|
| Sillikolloid P87 | Hoffman Mineral GmbH, Neuburg on the Danube, Germany | SiO2 (80%), Al2O3 (14%), Fe2O3 (<1%) | 0.25 g/cm3 | d97—6 μm, d50—1.5 μm | irregular round grains and lamellar stacks |
| Perlite, PEX-02/20 | Mining and Metal Works—Zakłady Górniczo-Metalowe Zębiec, Poland | SiO2 (65–75%), Al2O3 (10–18%), K2O + Na2O (6–9%), MgO + CaO (2–6%), Fe2O3 (1–5%) | 1–1.3 g/cm3 | 60% < 20 μm, 23.8%—20–32 μm, 5.4%—32–40 μm, 9.9%—40-63 μm, 0.6%—63–100 μm | the appearance of “crushed baubles” |
| Glass Beads MinTron 7™ | Rock Tron, Bristol, United Kingdom | SiO2 (48–60%), Al2O3 (20–30%), Fe2O3 (3–7%), alkali oxides (5–9%) | ~1.0 g/cm3 | d90—20–30 μm, d50—5–9 μm | predominantly regular spherical shapes |
| Sample | Surface Free Energy γs [mJ/m2] | Polar Component of Surface Free Energy γsp [mJ/m2] | Dispersion Component of Surface Free Energy γsd [mJ/m2] |
|---|---|---|---|
| i-PP | 28.10 | 2.76 | 25.25 |
| i-PP + 2.5% Sillikolloid | 31.42 | 1.28 | 30.15 |
| i-PP + 5% Sillikolloid | 30.78 | 1.09 | 26.69 |
| i-PP + 10% Sillikolloid | 30.22 | 1.74 | 28.48 |
| i-PP + 2.5% perlite | 30.98 | 1.21 | 29.77 |
| i-PP + 5% perlite | 30.01 | 0.38 | 29.63 |
| i-PP + 10% perlite | 30.91 | 0.62 | 30.30 |
| i-PP + 2.5% GB | 31.16 | 0.44 | 30.72 |
| i-PP + 5% GB | 32.11 | 0.48 | 31.62 |
| i-PP + 10% GB | 32.37 | 0.10 | 32.27 |
| i-PP O | 30.30 | 2.62 | 27.68 |
| i-PP O + 2.5% Sillikolloid | 29.24 | 1.07 | 28.17 |
| i-PP O + 5% Sillikolloid | 34.25 | 0.50 | 33.75 |
| i-PP O + 10% Sillikolloid | 32.58 | 1.12 | 31.46 |
| i-PP O + 2.5% perlite | 32.62 | 0.16 | 32.46 |
| i-PP O + 5% perlite | 31.01 | 0.11 | 30.91 |
| i-PP O + 10% perlite | 32.77 | 0.09 | 32.68 |
| i-PP O + 2.5% GB | 33.57 | 0.20 | 33.37 |
| i-PP O + 5% GB | 34.42 | 0.24 | 34.18 |
| i-PP O + 10% GB | 36.58 | 0.22 | 36.36 |
| Sample | Surface Free Energy γs [mJ/m2] | Polar Component of Surface Free Energy γsp [mJ/m2] | Dispersion Component of Surface Free Energy γsd [mJ/m2] |
|---|---|---|---|
| i-PP | 29.82 | 4.63 | 25.19 |
| i-PP + 2.5% Sillikolloid | 35.13 | 9.47 | 25.66 |
| i-PP + 5% Sillikolloid | 34.53 | 9.09 | 25.44 |
| i-PP + 10% Sillikolloid | 36.07 | 9.66 | 26.41 |
| i-PP + 2.5% perlite | 26.84 | 3.53 | 32.32 |
| i-PP + 5% perlite | 31.18 | 3.98 | 27.20 |
| i-PP + 10% perlite | 30.45 | 4.81 | 25.60 |
| i-PP + 2.5% GB | 36.44 | 9.60 | 26.84 |
| i-PP + 5% GB | 34.20 | 8.80 | 25.40 |
| i-PP + 10% GB | 40.82 | 4.77 | 36.06 |
| i-PP O | 35.06 | 5.07 | 30.0 |
| i-PP O + 2.5% Sillikolloid | 36.01 | 6.53 | 29.48 |
| i-PP O + 5% Sillikolloid | 37.03 | 2.73 | 34.30 |
| i-PP O + 10% Sillikolloid | 32.93 | 5.40 | 27.53 |
| i-PP O + 2.5% perlite | 37.00 | 3.86 | 33.14 |
| i-PP O + 5% perlite | 37.12 | 4.51 | 32.61 |
| i-PP O + 10% perlite | 35.41 | 6.05 | 29.36 |
| i-PP O + 2.5% GB | 38.76 | 10.50 | 28.26 |
| i-PP O + 5% GB | 41.91 | 12.78 | 29.13 |
| i-PP O + 10% GB | 38.80 | 10.65 | 28.15 |
| Sample | Unirradiated | 1 Month of UV Irradiation | ||||
|---|---|---|---|---|---|---|
| C 1s (% at.) | O 1s (% at.) | Si 2p (% at.) | C 1s (% at.) | O 1s (% at.) | Si 2p (% at.) | |
| i-PP | 97 | 2 | 1 | 93 | 5 | 2 |
| i-PP + 5% Sillikolloid | 98 | 2 | - | 93 | 5 | 2 |
| i-PP + 5% perlite | 99 | 1 | - | 91 | 6 | 3 |
| i-PP + 5% GB | 97 | 3 | - | 96 | 4 | - |
| i-PP O | 99 | 1 | - | 96 | 4 | - |
| i-PP O + 5% Sillikolloid | 99 | 1 | - | 93 | 6 | 1 |
| i-PP O + 5% perlite | 100 | 0 | - | 95 | 5 | - |
| i-PP O + 5% GB | 99 | 1 | - | 94 | 6 | - |
| Sample | Rq [nm] | Sample | Rq [nm] | ||
|---|---|---|---|---|---|
| 0 h | 1 Month-Irradiated | 0 h | 1 Month-Irradiated | ||
| i-PP | 13.8 | 17.4 | i-PP O | 32.1 | 30.0 |
| i-PP + 2.5% Sillikolloid | 44.9 | 45.5 | i-PP O + 2.5% Sillikolloid | 25.7 | 37.6 |
| i-PP + 5% Sillikolloid | 45.9 | 33.1 | i-PP O + 5% Sillikolloid | 20.0 | 41.0 |
| i-PP + 10% Sillikolloid | 76.0 | 56.7 | i-PP O + 10% Sillikolloid | 33.1 | 26.3 |
| i-PP + 2.5% perlite | 44.6 | 45.2 | i-PP O + 2.5% perlite | 32.7 | 47.3 |
| i-PP + 5% perlite | 29.5 | 42.1 | i-PP O + 5% perlite | 38.0 | 32.8 |
| i-PP + 10% perlite | 43.4 | 52.1 | i-PP O + 10% perlite | 50.6 | 62.8 |
| i-PP + 2.5% GB | 44.3 | 48.1 | i-PP O + 2.5% GB | 36.0 | 37.9 |
| i-PP + 5% GB | 42.9 | 23.6 | i-PP O + 5% GB | 36.4 | 44.7 |
| i-PP + 10% GB | 38.3 | 57.1 | i-PP O + 10% GB | 30.8 | 44.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chylińska, M.; Kaczmarek, H.; Moszyński, D.; Królikowski, B.; Kowalonek, J. Surface Studies of UV Irradiated Polypropylene Films Modified with Mineral Fillers Designed as Piezoelectric Materials. Polymers 2020, 12, 562. https://doi.org/10.3390/polym12030562
Chylińska M, Kaczmarek H, Moszyński D, Królikowski B, Kowalonek J. Surface Studies of UV Irradiated Polypropylene Films Modified with Mineral Fillers Designed as Piezoelectric Materials. Polymers. 2020; 12(3):562. https://doi.org/10.3390/polym12030562
Chicago/Turabian StyleChylińska, Marta, Halina Kaczmarek, Dariusz Moszyński, Bogusław Królikowski, and Jolanta Kowalonek. 2020. "Surface Studies of UV Irradiated Polypropylene Films Modified with Mineral Fillers Designed as Piezoelectric Materials" Polymers 12, no. 3: 562. https://doi.org/10.3390/polym12030562
APA StyleChylińska, M., Kaczmarek, H., Moszyński, D., Królikowski, B., & Kowalonek, J. (2020). Surface Studies of UV Irradiated Polypropylene Films Modified with Mineral Fillers Designed as Piezoelectric Materials. Polymers, 12(3), 562. https://doi.org/10.3390/polym12030562