Biodegradation Pattern of Glycopolymer Based on D-Mannose Oligomer and Hydroxypropyl Acrylate
Abstract
:1. Introduction
2. Materials and Methods
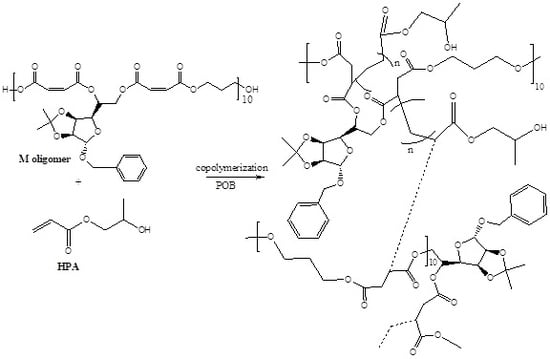
2.1. Glycopolymer Synthesis
2.2. DSC Analysis
2.3. Kinetic Isoconversional Methods for Activation Energy Assessment
2.4. Thermogravimetry
2.5. ATR-FTIR
2.6. Biodegradability Testing
2.7. Mathematical Models for Kinetics Modeling of Biodegradation Process
3. Results
3.1. Glycopolymer Synthesis. DSC Kinetic Analysis
3.2. Biodegradation Studies: Kinetics
3.3. TG Analysis of Glycopolymer Sample before and after Biodegradation
3.4. FTIR Analysis of Glycopolymer Sample before and after Biodegradation
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ghanbarzadeh, B.; Almasi, H. Biodegradable polymers. In Biodegradation-Life Science; Chamy, R., Rosenkranz, F., Eds.; InTech: London, UK, 2013; pp. 141–186. [Google Scholar]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, V.M. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- European Commission. A Circular Economy for Plastics, Insights from Research and Innovation to Inform Policy and Funding Decisions; De Smet, M., Linder, M., Eds.; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Harrison, J.P.; Boardman, C.; O’Callaghan, K.; Delort, A.M.; Song, J. Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review. R. Soc. Open Sci. 2018, 5, 171792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the great pacific garbage patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbanek, A.K.; Rymowicz, W.; Mironczuk, A.M. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 2018, 102, 7669–7678. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.D. Biodegradable polymers. Int. J. Appl. Chem. 2017, 13, 179–196. [Google Scholar]
- Adamcova, D.; Vaverkova, M. Degradation of biodegradable/degradable plastics in municipal solid-waste landfill. Pol. J. Environ. Stud. 2014, 23, 1071–1078. [Google Scholar]
- Vaterkova, M.; Toman, F.; Adamcova, D.; Kotovicova, J. Study of the biodegradability of degradable/biodegradable plastic material in a controlled composting environment. Ecol. Chem. Eng. S 2012, 19, 347–358. [Google Scholar]
- Wilfred, O.; Tai, H.; Marriot, R.; Liu, O.; Tverezovskiy, V.; Curling, S.; Tai, H.; Fan, Z.; Wang, W. Biodegradation of polylactic acid and starch composites in compost and soil. Int. J. Nano Res. 2018, 1, 1–11. [Google Scholar]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Insights on the aerobic biodegradation of polymers by analysis of evolved carbon dioxide in simulated composting conditions. Polym. Degrad. Stab. 2017, 137, 251–271. [Google Scholar] [CrossRef] [Green Version]
- Chinaglia, S.; Tosin, M.; Degli-Innocenti, F. Biodegradation rate of biodegradable plastics at molecular level. Polym. Degrad. Stab. 2018, 147, 237–244. [Google Scholar] [CrossRef]
- Haider, T.P.; Volker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Ding, D.; Zhao, S.; Zhu, H.; Kenttamaa, H.I.; Abu-Omar, M.M. Renewable thermoset polymers based on lignin and carbohydrate derived monomers. Green Chem. 2018, 5, 1131–1138. [Google Scholar] [CrossRef]
- Leja, K.; Lewandowicz, G. Polymers biodegradation and biodegradable polymers—A Review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- van Zee, M. Analytical methods for monitoring biodegradation processes. In Hankbook of Biodegradable Polymers: Synthesis, Characterization and Applications, 1st ed.; Lendlein, A., Sisson, A., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2011; pp. 263–281. [Google Scholar]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Microbial degradation, environmental and biotechnological perspectives. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef] [Green Version]
- Rodygin, K.; Werner, I.; Ananikov, V.P. A green and suitable route to carbohydrate vinyl ethers for accessing bioinspired materials with a unique microspherical morphology. ChemSusChem 2018, 11, 292–298. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Zhang, Y.; Zheng, Y.; Wen, X.; Bai, L.; Wu, Y. Hyperbranched Glycopolymers of 2-(α-D-Mannopyranose)Ethyl Methacrylate and N,N′-Methylenebisacrylamide: Synthesis, characterization and multivalent recognitions with Concanavalin A. Polymers 2018, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Minoda, M.; Otsubo, T.; Yamamoto, Y.; Zhao, J.; Honda, Y.; Tanaka, T.; Motoyanagi, J. The first synthesis of periodic and alternating glycopolymers by RAFT polymerization: A Novel synthetic pathway for glycosaminoglycan mimics. Polymers 2019, 11, 70. [Google Scholar] [CrossRef] [Green Version]
- Levit, M.; Zashikhina, N.; Vdovchenko, A.; Dobrodumov, A.; Zakharova, N.; Kashina, A.; Ruhl, E.; Lavrentieva, A.; Scheper, T.; Tennikova, T.; et al. Bio-inspired amphiphilic block-copolymers based on synthetic glycopolymer and poly(amino acid) as potential drug delivery systems. Polymers 2020, 12, 183. [Google Scholar] [CrossRef] [Green Version]
- Ionita, M.; Crica, L.E.; Voicu, S.I.; Dinescu, S.; Miculescu, F.; Costache, M.; Iovu, H. Synergistic effect of carbon nanotubes and graphene for high performance cellulose acetate membranes in biomedical applications. Carbohydr. Polym. 2018, 183, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Pană, A.M.; Popa, M.; Silion, M.; Sfȋrloagă, P.; Duchatel, L.; Bandur, G.; Rusnac, L.M. Novel semi-interpenetrating network hydrogels based on monnosaccharide oligomers with itaconic moiety: Synthesis and properties. Iran. Polym. J. 2017, 26, 743–751. [Google Scholar] [CrossRef]
- Pană, A.M.; Rusnac, L.M.; Bandur, G.; Şişu, E.; Badea, V.; Silion, M. Synthesis and characterization of new glycopolymers based on monosaccharides and maleic anhydride. I. Glucose derivatives. Mater. Plast. (Bucharest) 2010, 47, 28–34. [Google Scholar]
- Pană, A.M.; Rusnac, L.M.; Bandur, G.; Deleanu, C.; Bălan, M.; Silion, M. Synthesis and characterization of new glycopolymers based on monosaccharides and maleic anhydride. II. Mannose derivatives. Mater. Plast. (Bucharest) 2010, 47, 299–305. [Google Scholar]
- Ştefan, L.M.; Pană, A.M.; Pascariu, M.C.; Şişu, E.; Bandur, G.; Rusnac, L.M. Synthesis and characterization of a new methacrylic glycomonomer. Turk. J. Chem. 2011, 35, 757–767. [Google Scholar]
- Pană, A.M.; Gherman, V.; Sfîrloagă, P.; Bandur, G.; Ştefan, L.M.; Popa, M.; Rusnac, L.M. Thermal stability and biodegradation of novel D-mannose based glycopolymers. Polym. Test. 2012, 31, 384–392. [Google Scholar] [CrossRef]
- Ştefan, L.M.; Pană, A.M.; Bandur, G.; Martin, P.; Popa, M.; Rusnac, L.M. Thermal analysis of new glycopolymers derived from monosaccharides. J. Therm. Anal. Calorim. 2013, 111, 789–797. [Google Scholar] [CrossRef]
- Salagean, I.R.; Bandur, G.; Martin, P.; Lequart, V.; Rusnac, L.M. Synthesis and characterization of some carbohydrate based monomers. Rev. Chim. (Bucharest) 2009, 60, 905–908. [Google Scholar]
- Rusu, G.; Nitu, S.; Rusnac, L.; Bandur, G. Polymerization kinetic analysis of some glycerol and glucose based polymers. Macromol. Symp. 2015, 352, 51–58. [Google Scholar] [CrossRef]
- Rusu, G.; Joly, N.; Bandur, G.; Manoviciu, I.; Martin, P.; Rusnac, L. Inulin mixed esters crosslinked with 2-ethyl-hexyl-acrylate and their promotion as bio-based materials. J. Polym. Res. 2011, 18, 2495–2504. [Google Scholar] [CrossRef]
- Grandtner, G.; Joly, N.; Cavrot, J.P.; Granet, R.; Bandur, G.; Rusnac, L.M.; Martin, P.; Krausz, P. Synthesis of plastic films from inulin by acylation. Polym. Bull. 2005, 55, 235–241. [Google Scholar] [CrossRef]
- Pană, A.M.; Ştefan, L.M.; Bandur, G.; Sfîrloagă, P.; Gherman, V.; Silion, M.; Popa, M.; Rusnac, L.M. Novel glycopolymers based on D-mannose and methacrylates. Synthesis, thermal stability and biodegradability testing. J. Polym. Environ. 2013, 21, 981–994. [Google Scholar] [CrossRef]
- Pana, A.M.; Gherman, V.; Sfirloaga, P.; Rusu, G.; Bandur, G.; Popa, M.; Rusnac, L.M.; Dumitrel, G.A. Biodegradation studies on new glycopolymers derived from oligomeric D-mannose itaconates and 2-hydroxypropyl acrylate. Polym. Degrad. Stab. 2019, 167, 210–216. [Google Scholar] [CrossRef]
- Pană, A.M.; Rusnac, L.M.; Bandur, G.; Silion, M.; Deleanu, C.; Bălan, M. Novel D-glucose and D-mannose based oligomers. Synthesis and characterization. e-Polymers 2011, 11. [Google Scholar] [CrossRef] [Green Version]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Simulated Processes; Springer International Publishing: New York, NY, USA, 2015. [Google Scholar]
- Filimon, M.N.; Gotia, S.R.; Gotia, S.L.; Borozan, A.; Popescu, R.; Gherman, V.D. Bacteriological studies on river Timis with a role in evaluating pollution. Ann. Rom. Soc. Cell Biol. 2009, 14, 284–288. [Google Scholar]
- Oprean, L.; Lengyel, E.; Iancu, R. Monitoring and Evaluation of Timis river (Banat, Romania) water quality based on physicochemical and microbiological analysis. Transylv. Rev. Syst. Ecol. Res. 2013, 15, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Podaru, C.; Manea, F.; Vlaicu, I.; Patroescu, V.; Danielescu, C.; Burtica, G. Studies regarding surface water treatment using a microfiltration-ultrafiltration pilot plant. Environ. Eng. Manag. J. 2008, 7, 711–715. [Google Scholar] [CrossRef]
- Gordon, S.H.; Imam, S.H.; Shogren, R.L.; Govind, N.S.; Greene, R.V. A semiempirical model for predicting biodegradation profiles of individual polymers in starch–poly- (β-hydroxybutyrate-co-β-hydroxyvalerate) bioplastic. J. Appl. Polym. Sci. 2000, 76, 1767–1776. [Google Scholar] [CrossRef]
- Lagarias, J.C.; Reeds, J.A.; Wright, M.H.; Wright, P.E. Convergence properties of the nelder-mead simplex method in low dimensions. SIAM J. Optim. 1998, 9, 112–147. [Google Scholar] [CrossRef] [Green Version]
- Niaounakis, M. Biopolymers: Reuse, Recycling, and Disposal, 1st ed.; Andrew, W., Ed.; Elsevier: Waltham, MA, USA, 2013; pp. 107–148. [Google Scholar]






| Conversion, % | Activation Energy Ea, kJ/mol | |
|---|---|---|
| Kissinger-Akahira-Sunose | Flynn–Wall–Ozawa | |
| 10 | 88.78 | 89.84 |
| 20 | 94.85 | 96.94 |
| 30 | 95.02 | 96.82 |
| 40 | 98.74 | 100.42 |
| 50 | 106.40 | 108.45 |
| 60 | 110.95 | 112.41 |
| 70 | 112.10 | 113.97 |
| 80 | 113.54 | 114.2 |
| 90 | 118.95 | 120.78 |
| Average Activation Energy, kJ/mol | 104.37 | 105.98 |
| Parameter | a1 | a2 | a3 | a4 | a5 | a6 | a7 |
|---|---|---|---|---|---|---|---|
| 0.04478 | −0.0222 | 0.1296 | 0.1048 | 0.0024 | 1764.2996 | 1.6623 |
| Root-Mean Square Error, RMSE | Mean Square Error SD | Determination Coefficient R2 | Correlation Coefficient R | Relative Absolute Error, rAE |
|---|---|---|---|---|
| 1.5534 | 2.4133 | 0.9987 | 0.9942 | 0.0015 |
| Sample | Weight Loss, % | ||||
|---|---|---|---|---|---|
| 20–100 °C | 20–200 °C | 20–300 °C | 20–400 °C | 20–500 °C | |
| M_HPA2_before | 0.9573 | 3.0940 | 8.2544 | 56.0916 | 88.1286 |
| M_HPA2_after | 1.4948 | 4.3422 | 12.5916 | 57.4605 | 82.7941 |
| Sample | Inflexion Point Temperature, °C | |||
|---|---|---|---|---|
| M_HPA2_before | - | 368.8 | 386.5 | 434.3 |
| M_HPA2_after | 358 | 363.8 | 377.8 | 462.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pană, A.-M.; Ordodi, V.; Rusu, G.; Gherman, V.; Bandur, G.; Rusnac, L.-M.; Dumitrel, G.-A. Biodegradation Pattern of Glycopolymer Based on D-Mannose Oligomer and Hydroxypropyl Acrylate. Polymers 2020, 12, 704. https://doi.org/10.3390/polym12030704
Pană A-M, Ordodi V, Rusu G, Gherman V, Bandur G, Rusnac L-M, Dumitrel G-A. Biodegradation Pattern of Glycopolymer Based on D-Mannose Oligomer and Hydroxypropyl Acrylate. Polymers. 2020; 12(3):704. https://doi.org/10.3390/polym12030704
Chicago/Turabian StylePană, Ana-Maria, Valentin Ordodi, Gerlinde Rusu, Vasile Gherman, Geza Bandur, Lucian-Mircea Rusnac, and Gabriela-Alina Dumitrel. 2020. "Biodegradation Pattern of Glycopolymer Based on D-Mannose Oligomer and Hydroxypropyl Acrylate" Polymers 12, no. 3: 704. https://doi.org/10.3390/polym12030704
APA StylePană, A. -M., Ordodi, V., Rusu, G., Gherman, V., Bandur, G., Rusnac, L. -M., & Dumitrel, G. -A. (2020). Biodegradation Pattern of Glycopolymer Based on D-Mannose Oligomer and Hydroxypropyl Acrylate. Polymers, 12(3), 704. https://doi.org/10.3390/polym12030704