Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PCL-b-PDMS Diblock Copolymer
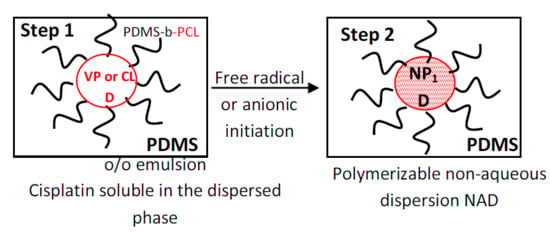
2.3. Non-Aqueous Emulsion Preparation and Characterization
2.4. Non-Aqueous Emulsion Polymerization and Characterization
2.5. Swelling Behavior in Aqueous Solutions
2.6. In Vitro Drug Release
2.7. In Vitro Hemolysis Assay
2.8. Cell Culture
2.9. Determination of the Cell Viability by the MTT Method
2.10. Apoptosis Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Non-Aqueous Emulsions and Dispersions
3.2. Swelling Behavior
3.3. In Vitro Release of Cis
3.4. In Vitro Evaluation of Nanoparticles Biocompatibility with the Blood Components
3.5. Determination of the Cell Viability by the MTT Method
3.6. Evaluation of Cell Apoptosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Petkar, K.C.; Chavhan, S.S.; Agatonovik-Kustrin, S.; Sawant, K.K. Nanostructured materials in drug and gene delivery: A review of the state of the art. Crit. Rev. Ther. Drug Carr. Syst. 2011, 28, 101–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Grund, S.; Bauer, M.; Fischer, D. Polymers in drug delivery-state of the art and future trends. Adv. Eng. Mater. 2011, 13, B61–B87. [Google Scholar] [CrossRef]
- Park, J.H.; Ye, M.; Park, K. Biodegradable polymers for microencapsulation of drugs. Molecules 2005, 10, 146–161. [Google Scholar] [CrossRef] [Green Version]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Heslinga, M.J.; Willis, G.M.; Sobczynski, D.J.; Thompson, A.J.; Eniola-Adefeso, O. One-step fabrication of agent-loaded biodegradable microspheroids for drug delivery and imaging applications. Colloids Surf. B 2014, 116, 55–62. [Google Scholar] [CrossRef]
- Del Rakhshayesh, A.R.; Akbarzadeh, A.; Alihemmati, A.; Nasrabadi, H.T.; Montaseri, A.; Davaran, S.; Abedelahi, A. Preparation and characterization of novel anti-inflammatory biological ahents based on piroxican-loaded poly-ε-caprolactone nano-particles for sustained NSAID delivery. Drug Deliv. 2020, 27, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Couvreur, P.; Kante, B.; Lenaerts, V.; Scailteur, V.; Roland, M.; Speiser, P. Tissue distribution of antitumor drugs associated with polyalkylcyanoacrylate nanoparticles. J. Pharm. Sci. 1980, 69, 199–202. [Google Scholar] [CrossRef]
- Dvorakova, G.; Haschick, R.; Klapper, M.; Mullen, K.; Biffis, A. Nonaqueous emulsion polymerization: A practical synthetic route for the production of molecularly imprinted nanospheres. J. Polym. Sci. A Polym. Chem. 2013, 51, 267–274. [Google Scholar] [CrossRef]
- Mbela, T.K.M.; Poupaert, J.H.; Dumont, P. Poly(diethylmethylidene malonate) nanoparticles as primaquine delivery system to liver. Int. J. Pharm. 1992, 79, 29–38. [Google Scholar] [CrossRef]
- Rajot, I.; Bone, S.; Graillat, C.; Hamaide, T. Nonionic nanoparticles by miniemulsion polymerization of vinyl acetate with oligocaprolactone macromonomer or Miglyol as hydrophobe. Application to the encapsulation of Indomethacin. Macromolecules 2003, 36, 7484–7490. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Zhang, H.; Jiang, G.; Kan, C. Synthesis and characterization of monodispersed P(St-co-DMAEMA) nanoparticles as pH-sensitive drug delivery system. Mater. Sci. Eng. C 2014, 45, 1–7. [Google Scholar] [CrossRef]
- Klapper, M.; Nenov, S.; Haschick, R.; Müller, K.; Müllen, K. Oil-in-oil emulsions: A unique tool for the formation of polymer nanoparticles. Acc. Chem. Res. 2008, 41, 1190–1201. [Google Scholar] [CrossRef]
- Weiss, C.K.; Landfester, K. Miniemulsion polymerization as a means to encapsulate organic and inorganic materials. Adv. Polym. Sci. 2010, 233, 185–236. [Google Scholar]
- Crespy, D.; Landfester, K. Making dry fertile: A practical tour of non-aqueous emulsions and miniemulsions, their preparation and some applications. Soft Matter 2011, 7, 11054–11064. [Google Scholar] [CrossRef]
- Ruppert, M.; Landfester, K.; Ziener, U. Anionic polymerization of cyclic ester and amide in miniemulsion: Synthesis and characterization of poly(ε-caprolactone) and poly(ε-caprolactone-co-ε-caprolactam) nanoparticles. J. Polym. Sci. A Polym. Chem. 2010, 48, 4929–4937. [Google Scholar] [CrossRef]
- Crespy, D.; Landfester, K. Synthesis of polyvinylpyrrolidone silver nanoparticles hybrid latex in non-aqueous miniemulsion at high temperature. Polymer 2009, 50, 1616–1620. [Google Scholar] [CrossRef]
- Hariri, K.; Al Akhrass, S.; Delaite, C.; Moireau, P.; Riess, G. Polymerizable oil-in-oil emulsions: Poly(vinyl pyrrolidone) dispersions in reactive PDMS medium. Polym. Int. 2007, 56, 1200–1205. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Block copolymers as polymeric stabilizers in non-aqueous emulsion polymerization. Polym. Int. 2011, 60, 1563–1573. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Stabilization of non-aqueous emulsions by poly(2-vinylpyridine)-b-poly(butadiene) block copolymers. Colloids Surf. A 2014, 458, 19–24. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. PEG 400/paraffin oil non-aqueous emulsions stabilized by PBut-block-P2VP block copolymers. J. Appl. Polym. Sci. 2014, 131, 41390. [Google Scholar] [CrossRef]
- Atanase, L.I.; Lerch, J.-P.; Riess, G. Water dispersibility of non-aqueous emulsions stabilized and viscosified by a poly(butadiene)-poly(2-vinylpyridine)-poly(ethylene oxide) PBut-P2VP-PEO triblock copolymer. Colloids Surf. A 2015, 464, 89–95. [Google Scholar] [CrossRef]
- Atanase, L.I.; Winninger, J.; Delaite, C.; Riess, G. Reversible addition-fragmentation chain transfert synthesis and micellar characteristics of biocompatible amphiphilic poly(vinyl acetate)-graft-poly(N-vinyl-2-pyrrolidone) copolymers. Eur. Polym. J. 2014, 53, 109–117. [Google Scholar] [CrossRef]
- Fares, M.M.; Assaf, S.M.; Abul-Haija, Y.M. Pectin grafted poly(N-vinylpyrrolidone): Optimization and in vitro controllable theophylline drug release. J. Appl. Polym. Sci. 2010, 117, 1945–1954. [Google Scholar] [CrossRef]
- Atanase, L.I.; Glaied, O.; Riess, G. Crystallization kinetics of PCL tagged with well-defined positional triazole defects generated by click-chemistry. Polymer 2011, 52, 3074–3081. [Google Scholar] [CrossRef]
- Witt, S.; Scheper, T.; Walter, J.G. Production of polycaprolactone nanoparticles with hydrodynamic diameters below 100 nm. Eng. Life Sci. 2019, 19, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.H.; Chang, J.Y. New insights into mechanisms of Cisplatin resistance: From tumor cell to microenvironment. Int. J. Mol. Sci. 2019, 20, 4136–4157. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.A.; Aquib, M.; Farooq, A.; Khan, D.H.; Maviah, M.B.J.; Filli, M.S.; Kesse, S.; Boakye-Yiadom, K.O.; Mavlyanova, R.; Parveen, A.; et al. Recent progress in nanotechnology-based novel drug delivery systems in designing of Cisplatin for cancer therapy: An overview. Artif. Cells Nanomedicine Biotechnol. 2019, 47, 1674–1692. [Google Scholar] [CrossRef] [Green Version]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.M.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Laville, N.; Aït-Aïssa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockert, J.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.; Villanueva, A. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Wlodkowic, D.; Skommer, J.; Darzynkiewicz, Z. Flow cytometry-based apoptosis detection. Methods Mol. Biol. 2009, 559, 19–32. [Google Scholar] [PubMed] [Green Version]
- Bacso, Z.; Everson, R.B.; Eliason, J.F. The DNA of Annexin V-binding apoptotic cells is highly fragmented. Cancer Res. 2000, 60, 4623–4628. [Google Scholar] [PubMed]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin v/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011, 50, e2597. [Google Scholar] [CrossRef]
- Siddik, Z.H. Cis: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [Green Version]
- Cepeda, V.; Fuertes, M.A.; Castilla, J.; Alonso, C.; Quevedo, C.; Perez, J.M. Biochemical mechanism of Cis cytotoxicity. Anticancer Agents Med. Chem. 2007, 7, 3–18. [Google Scholar] [CrossRef]
- Sudhakar, K.; Madhusudana Rao, K.; Sudhakar, P.; Chandra Babu, A.; Kumara Babu, P.; Subha, M.C.S.; Chowdoji Rao, K. Development of pH-sensitive polycaprolactone-based microspheres for in vitro release studies of Triprolidine Hydrochloride. Des. Monomers Polym. 2014, 17, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Cadinoiu, A.N.; Peptu, C.A.; Fache, B.; Chailan, J.F.; Popa, M. Microparticulated systems based on chitosan and poly(vinyl alcohol) with potential ophthalmic applications. J. Microencapsul. 2015, 32, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Burlui, V.; Popa, M.; Cadinoiu, A.N.; Stadoleanu, C.; Mihalache, G.; Zamaru, V.; Dârtu, L.; Folescu, E.; Rata, D.M. Physico-chemical characterization and in vitro hemolysis evaluation of titanium dioxide nanoparticles. Int. J. Med. Dent. 2015, 5, 124–130. [Google Scholar]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I. Biocompatible injectable polysaccharide materials for drug delivery. In Polysaccharide Carriers for Drug Delivery; Maiti, S., Jana, S., Eds.; Elsevier Woodhead Publishing: Witney, UK, 2019; pp. 127–154. [Google Scholar]
- Alupei, L.; Lisa, G.; Butnariu, A.; Desbrieres, J.; Cadinoiu, A.N.; Peptu, C.A.; Calin, G.; Popa, M. New folic acid-chitosan derivative based nanoparticles–potential applications in cancer therapy. Cell. Chem. Technol. 2017, 51, 631–648. [Google Scholar]
- Cregan, I.L.; Dharmarajan, A.M.; Fox, S.A. Mechanisms of Cisplatin-induced cell death in malignant mesothelioma cells: Role of inhibitor of apoptosis proteins (IAPs) and caspases. Int. J. Oncol. 2013, 42, 444–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Sample | Dispersed Phase | Cross-Linking Agent (wt%) a | DLS | |||
|---|---|---|---|---|---|---|
| Non-Aqueous Emulsion b | Non-Aqueous Dispersion c | |||||
| Droplet Size (nm) | PDI | Particle Size (nm) | PDI | |||
| NPs1 | VP | 1 | 122.5 ± 0.9 | 0.03 | 126.3 ± 1.0 | 0.03 |
| NPs2 | VP | 2 | 121.2 ± 2.0 | 0.01 | 128.3 ± 1.5 | 0.02 |
| NPs3 | VP | 3 | 117.7 ± 2.0 | 0.02 | 132.6 ± 0.5 | 0.05 |
| NPs4 | VP+Cis | 1 | 142.0 ± 1.8 | 0.04 | 148.0 ± 1.5 | 0.06 |
| NPs5 | CL | - | 120.9 ± 3.0 | 0.04 | 132.1 ± 0.8 | 0.05 |
| NPs6 | CL+Cis | - | 137.5 ± 1.8 | 0.06 | 148.8 ± 1.2 | 0.07 |
| Sample | n | K | R2 |
|---|---|---|---|
| NPs4 | 0.22 | 1.65 | 0.957 |
| NPs6 | 0.60 | 2.54 | 0.972 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization. Polymers 2020, 12, 1018. https://doi.org/10.3390/polym12051018
Daraba OM, Cadinoiu AN, Rata DM, Atanase LI, Vochita G. Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization. Polymers. 2020; 12(5):1018. https://doi.org/10.3390/polym12051018
Chicago/Turabian StyleDaraba, Oana Maria, Anca Niculina Cadinoiu, Delia Mihaela Rata, Leonard Ionut Atanase, and Gabriela Vochita. 2020. "Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization" Polymers 12, no. 5: 1018. https://doi.org/10.3390/polym12051018
APA StyleDaraba, O. M., Cadinoiu, A. N., Rata, D. M., Atanase, L. I., & Vochita, G. (2020). Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization. Polymers, 12(5), 1018. https://doi.org/10.3390/polym12051018