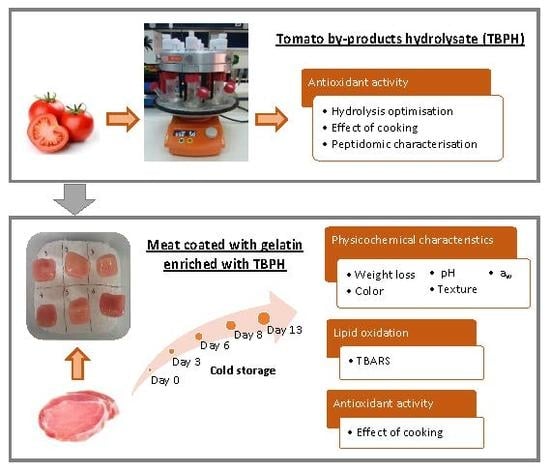
Effect of Gelatin Coating Enriched with Antioxidant Tomato By-Products on the Quality of Pork Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of the Tomato By-Products Hydrolysate
2.2.1. Optimisation of Enzymatic Hydrolysis
2.2.2. Cooking of TBPH
2.2.3. Peptidomic Characterisation
Size-Exclusion Chromatography (SEC)
Reversed-Phase High Performance Liquid Chromatography (RP-HPLC)
Peptide Identification by Tandem Mass Spectrometry
In Silico Analysis
2.3. Preparation of Pork Loin Samples Coated with Gelatin and Gelatin Enriched with TBPH
2.3.1. Gelatin Solution Preparation and Enrichment with TBPH
2.3.2. Gelatin Coating of Pork Loin
2.4. Antioxidant Activity
2.4.1. DPPH Radical Scavenging Activity
2.4.2. Ferric-reducing Antioxidant Power (FRAP)
2.4.3. ABTS Radical Scavenging Capacity
2.5. Weight Loss, pH, Water Activity and Colour Measurements
2.6. Texture Profile Analysis
2.7. Lipid Oxidation
2.8. Statistical Analysis
3. Results and Discussion
3.1. TBPH Characterisation
3.1.1. Optimisation of Hydrolysis Time in the Preparation of TBPH
3.1.2. Effect of Cooking on Antioxidant Activity of TBPH
3.1.3. Peptidomic Characterisation
Antioxidant Fractionation by SEC and RP-HPLC
Peptide Identification and In Silico Analysis
3.2. Coating of Fresh Pork Loin with Gelatin Enriched with Tomato By-Products Hydrolysate
3.2.1. Physicochemical Characteristics
3.2.2. Effect of Coating and Enriched Coating on Meat Lipid Oxidation
3.2.3. Effect of Cooking on Antioxidant Activity of Coated Meats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antoniewski, M.N.; Barringer, S.A. Meat shelf-life and extension using collagen/gelatin coatings: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Valdes, A.; Beltran, A.; Garrigós, M.C. Gelatin-based films and coatings for food packaging applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Etxabide, A.; Uranga, J.; Guerrero, P.; De la Caba, K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocoll. 2017, 68, 192–198. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Antoniewski, M.N.; Barringer, S.A.; Knipe, C.L.; Zerby, H.N. Effect of a gelatin coating on the shelf life of fresh meat. J. Food Sci. 2007, 72, E382–E387. [Google Scholar] [CrossRef]
- Herring, J.L.; Jonnalongadda, S.C.; Narayanan, V.C.; Coleman, S.M. Oxidative stability of gelatin coated pork at refrigerated storage. Meat Sci. 2010, 85, 651–656. [Google Scholar] [CrossRef]
- Jridi, M.; Mora, L.; Souissi, N.; Aristoy, M.C.; Nasri, M.; Toldrá, F. Effects of active gelatin coated with henna (L. inermis) extract on beef meat quality during chilled storage. Food Control 2018, 84, 238–245. [Google Scholar] [CrossRef]
- Li, J.H.; Miao, J.; Wu, J.L.; Chen, S.F.; Zhang, Q.Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, R.; Jridi, M.; Kchaou, H.; Nasreddine, B.; Karbowiak, T.; Debeaufort, F.; Nasri, M. Composite bioactive films based on smooth-hound viscera proteins and gelatin: Physicochemical characterization and antioxidant properties. Food Hydrocoll. 2018, 74, 176–186. [Google Scholar] [CrossRef]
- Hanani, Z.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Chen, M.; Jiang, S.; Cheng, J.; Li, X.; Zhang, M.; Jiang, S. Gelatin/zein fiber mats encapsulated with resveratrol: Kinetics, antibacterial activity and application for pork preservation. Food Hydrocoll. 2020, 101, 105577. [Google Scholar] [CrossRef]
- Moayedi, A.; Hashemi, M.; Safari, M. Valorization of tomato waste proteins through production of antioxidant and antibacterial hydrolysates by proteolytic Bacillus subtilis: Optimization of fermentation conditions. J. Food Sci. Technol. 2016, 53, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moayedi, A.; Mora, L.; Aristoy, M.C.; Safari, M.; Hashemi, M.; Toldrá, F. Peptidomic analysis of antioxidant and ACE-inhibitory peptides obtained from tomato waste proteins fermented using Bacillus subtilis. Food Chem. 2018, 250, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Meshginfar, N.; Mahoonak, A.S.; Hosseinian, F.; Ghorbani, M.; Tsopmo, A. Production of antioxidant peptide fractions from a by-product of tomato processing: Mass spectrometry identification of peptides and stability to gastrointestinal digestion. J. Food Sci. Technol. 2018, 55, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [Green Version]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the improved discovery and design of functional peptides: Common features of diverse classes permit generalized prediction of bioactivity. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G.P. Open Source Drug Discovery Consortium. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v. 2—A server for in silico prediction of allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Huang, S.J.; Mau, J.L. Antioxidant properties of hot water extracts from Agrocybe cylindracea. Food Chem. 2006, 98, 670–677. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Salih, A.M.; Smith, D.M.; Price, J.F.; Dawson, L.E. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987, 66, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Xiong, Y.L. Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J. Agric. Food Chem. 2006, 54, 6059–6068. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Jang, H.L.; Liceaga, A.M.; Yoon, K.Y. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Foods 2016, 20, 433–442. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Zhang, W.G.; Kang, Z.L.; Zhou, G.H.; Xu, X.L. Stability of an antioxidant peptide extracted from Jinhua ham. Meat Sci. 2014, 96, 783–789. [Google Scholar] [CrossRef]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef]
- Damgaard, T.; Lametsch, R.; Otte, J. Antioxidant capacity of hydrolyzed animal by-products and relation to amino acid composition and peptide size distribution. J. Food Sci. Technol. 2015, 52, 6511–6519. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides-opportunities for designing future foods. Curr. Pharm Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [Green Version]
- Chi, C.F.; Wang, B.; Wang, Y.M.; Zhang, B.; Deng, S.G. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Characterisation of the antioxidant peptide AEEEYPDL and its quantification in Spanish dry-cured ham. Food Chem. 2018, 258, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lv, J.; Gong, J.; Xiao, G.; Zhu, R.; Li, L.; Qiu, J. Secondary structures and their effects on antioxidant capacity of antioxidant peptides in yogurt. Int. J. Food Prop. 2018, 21, 2167–2180. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [Green Version]
- Finch, C.A.; Jobling, A. The physical properties of gelatin. In The Science and Technology of Gelatin; Ward, A.G., Courts, A., Eds.; Academic Press: London, UK, 1977; pp. 249–294. [Google Scholar]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef]
- Insausti, K.; Beriain, M.J.; Purroy, A.; Alberti, P.; Gorraiz, C.; Alzueta, M.J. Shelf life of beef from local Spanish cattle breeds stored under modified atmosphere. Meat Sci. 2001, 57, 273–281. [Google Scholar] [CrossRef]
- López-Caballero, M.E.; Pérez-Mateos, M.; Borderias, J.A.; Montero, P. Extension of the shelf life of prawns (Penaeus japonicus) by vacuum packaging and high-pressure treatment. J. Food Prot. 2000, 63, 1381–1388. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Liu, S.; Wang, Y. Effects of antimicrobial coating from catfish skin gelatin on quality and shelf life of fresh white shrimp (Penaeus vannamei). J. Food Sci. 2011, 76, M204–M209. [Google Scholar] [CrossRef]
- Nowak, B.; Von Mueffling, T.; Grotheer, J.; Klein, G.; Watkinson, B.M. Energy content, sensory properties, and microbiological shelf life of German Bologna-type sausages produced with citrate or phosphate and with inulin as fat replacer. J. Food Sci. 2007, 72, S629–S638. [Google Scholar] [CrossRef]
- Marggrander, K.; Hofmann, K. Reduction of freezer burn and loss on drying during long term storage of pork with gelatin spray solution. Fleischwirtschaft 1997, 77, 19–20. [Google Scholar]
- Giménez, B.; Gómez-Estaca, J.; Alemán, A.; Gómez-Guillén, M.C.; Montero, M.P. Improvement of the antioxidant properties of squid skin gelatin films by the addition of hydrolysates from squid gelatin. Food Hydrocoll. 2009, 23, 1322–1327. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef] [PubMed]
- Santé-Lhoutellier, V.; Astruc, T.; Marinova, P.; Greve, E.; Gatellier, P. Effect of meat cooking on physicochemical state and in vitro digestibility of myofibrillar proteins. J. Agric. Food Chem. 2008, 56, 1488–1494. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Vhangani, L.N.; Van Wyk, J. Antioxidant activity of Maillard reaction products (MRPs) in a lipid-rich model system. Food Chem. 2016, 208, 301–308. [Google Scholar] [CrossRef]






| RP-HPLC Fractions | Peptide Sequence | Peptide Ranker Score | Hydrophobicity | Hydrophilicity | Steric Hindrance | Amphipathicity | Toxicity Prediction | Allergenicity Prediction |
|---|---|---|---|---|---|---|---|---|
| 3 | GGPAAGCCCRDCCVE | 0.95 | −0.10 | 0.10 | 0.62 | 0.25 | Toxic | Allergen |
| GGFGGMC | 0.95 | 0.22 | −0.69 | 0.64 | 0.00 | Non-toxic | Non-allergen | |
| LLIVILFLTIC | 0.70 | 0.48 | −1.64 | 0.62 | 0.00 | Non-toxic | Non-allergen | |
| PAAQPGC | 0.68 | −0.02 | −0.26 | 0.53 | 0.18 | Non-toxic | Non-allergen | |
| EFTCPNC | 0.66 | −0.12 | −0.24 | 0.61 | 0.18 | Non-toxic | Allergen | |
| EQAPACAMG | 0.66 | −0.02 | −0.07 | 0.60 | 0.28 | Non-toxic | Non-allergen | |
| GCNGEPC | 0.65 | −0.13 | 0.17 | 0.63 | 0.18 | Non-toxic | Allergen | |
| CCQCSYA | 0.55 | −0.08 | −0.76 | 0.61 | 0.18 | Toxic | Non-allergen | |
| 12-13 | SCPCCGT | 0.89 | −0.03 | −0.44 | 0.57 | 0.00 | Toxic | Allergen |
| LPSECGFC | 0.87 | 0.05 | −0.38 | 0.59 | 0.16 | Non-toxic | Allergen | |
| PGGAGPC | 0.81 | 0.09 | −0.21 | 0.56 | 0.00 | Non-toxic | Non-allergen | |
| CLATCFCPN | 0.78 | 0.07 | −0.89 | 0.58 | 0.00 | Non-toxic | Allergen | |
| VPSGCFEGGAGNC | 0.77 | 0.04 | −0.23 | 0.63 | 0.10 | Non-toxic | Non-allergen | |
| SEYCCCSC | 0.77 | −0.12 | −0.34 | 0.61 | 0.16 | Toxic | Allergen | |
| QCGEGMC | 0.69 | −0.09 | −0.01 | 0.68 | 0.36 | Non-toxic | Allergen | |
| CSQGEGSYEGPLG | 0.66 | −0.10 | 0.13 | 0.62 | 0.29 | Non-toxic | Non-allergen | |
| SGADPAC | 0.64 | −0.05 | 0.19 | 0.57 | 0.00 | Non-toxic | Allergen | |
| SGGGACSDTGACTPAR | 0.58 | −0.12 | 0.14 | 0.59 | 0.15 | Non-toxic | Allergen | |
| GRGGGAC | 0.54 | −0.12 | 0.21 | 0.65 | 0.35 | Non-toxic | Non-allergen | |
| ANGAAGC | 0.54 | 0.07 | −0.33 | 0.61 | 0.00 | Non-toxic | Allergen | |
| 16-17 | DPQYPPGPPAF | 0.88 | −0.07 | −0.19 | 0.53 | 0.11 | Non-toxic | Non-allergen |
| CWQDPSMDMH | 0.84 | −0.19 | −0.10 | 0.58 | 0.27 | Non-toxic | Non-allergen | |
| GKCECGQCTCFP | 0.83 | −0.13 | −0.06 | 0.62 | 0.52 | Toxic | Allergen | |
| IHGGGWC | 0.80 | 0.17 | −0.96 | 0.55 | 0.21 | Non-toxic | Non-allergen | |
| AIILFFVCILV | 0.78 | 0.53 | −1.68 | 0.65 | 0.00 | Non-toxic | Non-allergen | |
| NPGPPGT | 0.71 | −0.10 | −0.03 | 0.53 | 0.00 | Non-toxic | Non-allergen | |
| GPSPQAC | 0.70 | −0.09 | −0.14 | 0.54 | 0.18 | Non-toxic | Non-allergen | |
| GSPGEPM | 0.69 | −0.06 | 0.29 | 0.58 | 0.18 | Non-toxic | Allergen | |
| SLALYLP | 0.68 | 0.22 | −1.13 | 0.53 | 0.00 | Non-toxic | Allergen | |
| LPGGARC | 0.67 | −0.10 | −0.04 | 0.58 | 0.35 | Non-toxic | Non-allergen | |
| SPGRGGG | 0.64 | −0.21 | 0.47 | 0.61 | 0.35 | Non-toxic | Non-allergen | |
| DCSDGSDEKNCDCG | 0.64 | −0.38 | 1.13 | 0.67 | 0.35 | Toxic | Non-allergen | |
| GPELPPVP | 0.63 | 0.04 | −0.04 | 0.50 | 0.16 | Non-toxic | Allergen | |
| MGDTGPCG | 0.63 | −0.02 | 0.04 | 0.64 | 0.00 | Non-toxic | Non-allergen | |
| GGGSPPA | 0.61 | 0.05 | −0.03 | 0.54 | 0.00 | Non-toxic | Non-allergen | |
| LCSWPGGQSSGVPG | 0.60 | 0.04 | −0.47 | 0.58 | 0.09 | Non-toxic | Non-allergen | |
| GCCILYS | 0.58 | 0.18 | −1.09 | 0.63 | 0.00 | Toxic | Allergen | |
| GGGGGHP | 0.56 | 0.05 | −0.07 | 0.54 | 0.21 | Non-toxic | Non-allergen | |
| NPSLSGC | 0.56 | −0.07 | −0.29 | 0.57 | 0.00 | Non-toxic | Allergen | |
| SGQGTPFSYSVPG | 0.53 | −0.01 | −0.43 | 0.59 | 0.10 | Non-toxic | Non-allergen | |
| YGGGGGR | 0.53 | −0.13 | 0.10 | 0.68 | 0.35 | Non-toxic | Non-allergen | |
| APKRQSPLP | 0.53 | −0.36 | 0.47 | 0.52 | 0.82 | Non-toxic | Non-allergen | |
| ICCGIGAY | 0.51 | 0.27 | −1.05 | 0.65 | 0.00 | Toxic | Non-allergen | |
| AGFGAAN | 0.51 | 0.15 | −0.54 | 0.63 | 0.00 | Non-toxic | Allergen | |
| PSEPTTFGPT | 0.51 | −0.09 | −0.04 | 0.53 | 0.13 | Non-toxic | Non-allergen |
| Parameter | Sample | Storage Time (days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 8 | 13 | |||
| Weigth loss (%) | Meat | 2.09 ± 0.52 Bb | 2.98 ± 1.29 Bb | 3.18 ± 0.59 Bb | 8.08 ± 3.35 Aa | ||
| Meat + Gelatin-TBPH | 4.39 ± 0.41 Ab | 7.19 ± 1.08 Aa | 6.22 ± 0.80 Aab | 7.73 ± 3.15 Aa | |||
| pH | Meat | 5.60 ± 0.02 Ab | 5.46 ± 0.11 Ab | 5.50 ± 0.23 Ab | 5.83 ± 0.36 Ab | 7.12 ± 0.28 Aa | |
| Meat + Gelatin-TBPH | 5.60 ± 0.02 Aa | 5.57 ± 0.13 Aa | 5.63 ± 0.50 Aa | 5.44 ± 0.04 Aa | 5.88 ± 0.21 Ba | ||
| aw | Meat | 0.98 ± 0.00 Aa | 0.98 ± 0.00 Aa | 0.98 ± 0.00 Aa | 0.98 ± 0.00 Aa | 0.98 ± 0.00 Aa | |
| Meat + Gelatin-TBPH | 0.98 ± 0.00 Aa | 0.99 ± 0.00 Aa | 0.99 ± 0.00 A | 0.98 ± 0.00 Aa | 0.99 ± 0.00 Aa | ||
| Colour | L* | Meat | 43.37 ± 2.40 Ac | 44.13 ± 1.70 Bbc | 42.68 ± 1.60 Bc | 47.11 ± 2.37 Ba | 45.44 ± 3.91 Bb |
| Meat + Gelatin-TBPH | 43.37 ± 2.40 Ac | 50.42 ± 1.44 Ab | 52.00 ± 1.41 Aa | 51.56 ± 2.15 Aa | 51.73 ± 2.39 Aa | ||
| C* | Meat | 7.21 ± 0.85 Abc | 6.92 ± 1.01 Ac | 6.97 ± 0.87 Ac | 7.55 ± 0.81 Ab | 9.47 ± 1.08 Aa | |
| Meat + Gelatin-TBPH | 7.21 ± 0.85 Aa | 7.28 ± 0.73 Aa | 7.09 ± 0.91 Aa | 7.38 ± 1.00 Aa | 7.53 ± 0.74 Ba | ||
| h* | Meat | 86.69 ± 6.42 Ab | 83.11 ± 4.82 Ac | 79.93 ± 3.37 Bd | 83.34 ± 5.73 Bc | 91.54 ± 6.75 Aa | |
| Meat + Gelatin-TBPH | 86.69 ± 6.42 Ab | 80.30 ± 7.77 Ac | 86.38 ± 5.81 Ab | 88.87 ± 7.44 Aab | 91.10 ± 6.57 Aa | ||
| ΔΕ* | Meat | 3.50 ± 1.62 Bbc | 2.77 ± 1.46 Bc | 4.51 ± 2.59 Bb | 5.91 ± 2.89 Ba | ||
| Meat + Gelatin-TBPH | 7.26 ± 2.30 Aa | 8.80 ± 2.47 Aa | 8.41 ± 3.44 Aa | 8.50 ± 2.71 Aa | |||
| TPA | Hardness (N) | Meat | 150.50 ± 34.48 Ab | 158.30 ± 24.90 Aab | 164.07 ± 39.23 Aab | 196.72 ± 45.67 Aa | 156.46 ± 19.04 Bab |
| Meat + Gelatin-TBPH | 150.50 ± 34.48 Ab | 145.02 ± 37.70 Ab | 192.07 ± 22.67 Aab | 154.38 ± 60.86 Ab | 210.48 ± 31.35 Aa | ||
| Elasticity | Meat | 0.35 ± 0.04 Aab | 0.34 ± 0.03 Aab | 0.32 ± 0.05 Aab | 0.29 ± 0.03 Ab | 0.39 ± 0.09 Aa | |
| Meat + Gelatin-TBPH | 0.35 ± 0.04 Aa | 0.31 ± 0.03 Aab | 0.34 ± 0.05 Aa | 0.29 ± 0.02 Ab | 0.32 ± 0.04 Aab | ||
| Adhesiveness (N·s) | Meat | −0.38 ± 0.14 Ab | −0.51 ± 0.11 Ab | −0.74 ± 0.37 Ab | −0.60 ± 0.24 Ab | −2.00 ± 1.48 Aa | |
| Meat + Gelatin-TBPH | −0.38 ± 0.14 Ab | −0.22 ± 0.11 Bb | −0.31 ± 0.13 Bb | −0.39 ± 0.28 Ab | −0.89 ± 0.38 Aa | ||
| Cohesiveness | Meat | 0.33 ± 0.07 Aab | 0.31 ± 0.05 Ab | 0.40 ± 0.07 Aa | 0.39 ± 0.06 Aab | 0.41 ± 0.07 Aa | |
| Meat + Gelatin-TBPH | 0.33 ± 0.07 Ac | 0.31 ± 0.05 Ac | 0.40 ± 0.05 Ab | 0.33 ± 0.05 Ac | 0.45 ± 0.03 Aa | ||
| Chewiness (N) | Meat | 17.50 ± 7.85 Aa | 17.05 ± 6.02 Aa | 22.08 ± 10.91 Aa | 23.59 ± 10.35 Aa | 25.02 ± 9.06 Aa | |
| Meat + Gelatin-TBPH | 17.50 ± 7.85 Ab | 14.49 ± 6.02 Ab | 26.60 ± 9.95 Aa | 15.38 ± 8.00 Ab | 30.07 ± 5.43 Aa | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego, M.; Arnal, M.; Talens, P.; Toldrá, F.; Mora, L. Effect of Gelatin Coating Enriched with Antioxidant Tomato By-Products on the Quality of Pork Meat. Polymers 2020, 12, 1032. https://doi.org/10.3390/polym12051032
Gallego M, Arnal M, Talens P, Toldrá F, Mora L. Effect of Gelatin Coating Enriched with Antioxidant Tomato By-Products on the Quality of Pork Meat. Polymers. 2020; 12(5):1032. https://doi.org/10.3390/polym12051032
Chicago/Turabian StyleGallego, Marta, Milagros Arnal, Pau Talens, Fidel Toldrá, and Leticia Mora. 2020. "Effect of Gelatin Coating Enriched with Antioxidant Tomato By-Products on the Quality of Pork Meat" Polymers 12, no. 5: 1032. https://doi.org/10.3390/polym12051032
APA StyleGallego, M., Arnal, M., Talens, P., Toldrá, F., & Mora, L. (2020). Effect of Gelatin Coating Enriched with Antioxidant Tomato By-Products on the Quality of Pork Meat. Polymers, 12(5), 1032. https://doi.org/10.3390/polym12051032