Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering
Abstract
:1. Introduction
2. Cartilage Types, Properties, and Formation
3. Agarose Properties
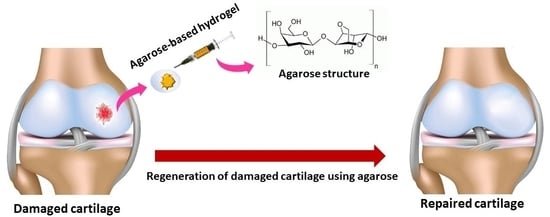
4. Agarose in Cartilage Regeneration
5. Future Perspective and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Robbins, S.; Abram, F.; Boily, M.; Pelletier, J.-P.; Martel-Pelletier, J. Relationship between alignment and cartilage thickness in patients with non-traumatic and post-traumatic knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Saeb, M.R.; Zarrintaj, P.; Kundu, S.C.; Khademhosseini, A.J. Silk fibroin scaffolds for common cartilage injuries: Possibilities for future clinical applications. Eur. Polym. J. 2019, 115, 251–267. [Google Scholar] [CrossRef]
- Orman, O.; Ozturk, K.; Baydar, M.; Guneren, E.; Taslidere, E.; Orman, M.; Ozel, O. Influence of articular arthroscopy-like washout on fracture healing of intra-articular fractures. Anim. Exp. 2020, 3, 18–29. [Google Scholar] [CrossRef]
- Khalili, R.; Zarrintaj, P.; Jafari, S.H.; Vahabi, H.; Saeb, M.R. Electroactive poly (p-phenylene sulfide)/r-Graphene Oxide/Chitosan as a novel potential candidate for tissue engineering. Int. J. Biol. Macromol. 2020, 154, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Hasanzadeh, R.; Azdast, T.; Abdollahi, H.; Zarrintaj, P.; Saeb, M.R. Piezoelectric Performance of Microcellular Polypropylene Foams Fabricated Using Foam Injection Molding as a Potential Scaffold for Bone Tissue Engineering. J. Macromol. Sci. Part B 2020, 1–14. [Google Scholar] [CrossRef]
- Saberi, A.; Jabbari, F.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Electrically Conductive Materials: Opportunities and Challenges in Tissue Engineering. Biomolecules 2019, 9, 448. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Saeb, M.R.; Ramakrishna, S.; Mozafari, M. Biomaterials selection for neuroprosthetics. Curr. Opin. Biomed. Eng. 2018, 6, 99–109. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Saeb, M.R.; Jafari, S.H.; Mozafari, M. Application of compatibilized polymer blends in biomedical fields. In Compatibilization of Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2020; pp. 511–537. [Google Scholar]
- Zarrintaj, P.; Bakhshandeh, B.; Saeb, M.R.; Sefat, F.; Rezaeian, I.; Ganjali, M.R.; Ramakrishna, S.; Mozafari, M. Oligoaniline-based conductive biomaterials for tissue engineering. Acta Biomater. 2018, 72, 16–34. [Google Scholar] [CrossRef]
- García-Martínez, L.; Campos, F.; Godoy-Guzmán, C.; Sánchez-Quevedo, M.D.; Garzón, I.; Alaminos, M.; Campos, A.; Carriel, V. Encapsulation of human elastic cartilage-derived chondrocytes in nanostructured fibrin-agarose hydrogels. Histochem. Cell Biol. 2017, 147, 83–95. [Google Scholar] [CrossRef]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in biomedical engineering: A critical review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef]
- Bagheri, B.; Zarrintaj, P.; Samadi, A.; Zarrintaj, R.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M.; Park, O.O.; Kim, Y.-C. Tissue engineering with electrospun electro-responsive chitosan-aniline oligomer/polyvinyl alcohol. Int. J. Biol. Macromol. 2020, 147, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Zarrintaj, P.; Surwase, S.S.; Baheiraei, N.; Saeb, M.R.; Mozafari, M.; Kim, Y.-C.; Park, O.O. Self-gelling electroactive hydrogels based on chitosan-aniline oligomers/agarose for neural tissue engineering with on-demand drug release. Colloids Surf. B Biointerfaces 2019, 184, 110549. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, R.; Zarrintaj, P.; Kamrava, S.K.; Bagher, Z.; Farhadi, M.; Heidari, F.; Komeili, A.; Gutiérrez, T.J.; Saeb, M.R. Conductive hydrogels based on agarose/alginate/chitosan for neural disorder therapy. Carbohydr. Polym. 2019, 224, 115161. [Google Scholar] [CrossRef] [PubMed]
- Atoufi, Z.; Zarrintaj, P.; Motlagh, G.H.; Amiri, A.; Bagher, Z.; Kamrava, S.K. A novel bio electro active alginate-aniline tetramer/agarose scaffold for tissue engineering: Synthesis, characterization, drug release and cell culture study. Polym. Ed. 2017, 28, 1617–1638. [Google Scholar] [CrossRef]
- Bagher, Z.; Atoufi, Z.; Alizadeh, R.; Farhadi, M.; Zarrintaj, P.; Moroni, L.; Setayeshmehr, M.; Komeili, A.; Kamrava, S.K. Conductive hydrogel based on chitosan-aniline pentamer/gelatin/agarose significantly promoted motor neuron-like cells differentiation of human olfactory ecto-mesenchymal stem cells. Mater. Sci. Eng. C 2019, 101, 243–253. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Urbanska, A.M.; Gholizadeh, S.S.; Goodarzi, V.; Saeb, M.R.; Mozafari, M. A facile route to the synthesis of anilinic electroactive colloidal hydrogels for neural tissue engineering applications. J. Colloid Interface Sci. 2018, 516, 57–66. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Kock, L.M.; Van Donkelaar, C.C.; Ito, K. Agarose concentration and TGF-B3 supplementation influence matrix deposition in engineered cartilage constructs. In Proceedings of the ASME 2011 Summer Bioengineering Conference, Farmington, PA, USA, 22–25 June 2011; pp. 879–880. [Google Scholar]
- Zarrintaj, P.; Moghaddam, A.S.; Manouchehri, S.; Atoufi, Z.; Amiri, A.; Amirkhani, M.A.; Nilforoushzadeh, M.A.; Saeb, M.R.; Hamblin, M.R.; Mozafari, M. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine 2017, 12, 2403–2422. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A novel electroactive agarose-aniline pentamer platform as a potential candidate for neural tissue engineering. Sci. Rep. 2017, 7, 17187. [Google Scholar] [CrossRef] [Green Version]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan–Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef]
- Grolman, J.M.; Singh, M.; Mooney, D.J.; Eriksson, E.; Nuutila, K. Antibiotic-containing agarose hydrogel for wound and burn care. J. Burn Care Res. 2019, 40, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Rubin, A.L.; Asina, S.; Smith, B.; Stenzel, K. Agarose Coated Agarose Beads Containing Cancer Cells that Produce Material which Suppresses Cancer Cell Proliferation. U.S. Patent 5,888,497, 30 March 1999. [Google Scholar]
- Guo, X.; Chen, Y.; Ji, W.; Chen, X.; Li, C.; Ge, R. Enrichment of cancer stem cells by agarose multi-well dishes and 3D spheroid culture. Cell Tissue Res. 2019, 375, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, M.; Qin, L.; Mahmoodi, P.; Dong, G. Osmosis effect on protein sustained release of Agarose hydrogel for anti-friction performance. Tribol. Int. 2019, 132, 108–117. [Google Scholar] [CrossRef]
- Hasan, M.L.; Padalhin, A.R.; Kim, B.; Lee, B.T. Preparation and evaluation of BCP-CSD-agarose composite microsphere for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2263–2272. [Google Scholar] [CrossRef]
- Carriel, V.; Vizcaíno-López, G.; Chato-Astrain, J.; Durand-Herrera, D.; Alaminos, M.; Campos, A.; Sánchez-Montesinos, I.; Campos, F. Scleral surgical repair through the use of nanostructured fibrin/agarose-based films in rabbits. Exp. Eye Res. 2019, 186, 107717. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, A. Multi-featured macroporous agarose–alginate cryogel: Synthesis and characterization for bioengineering applications. Macromol. Biosci. 2011, 11, 22–35. [Google Scholar] [CrossRef]
- Pearl, G.S.; Check, I.J.; Hunter, R.L. Agarose electrophoresis and immunonephelometric quantitation of cerebrospinal fluid immunoglobulins: Criteria for application in the diagnosis of neurologic disease. Am. J. Clin. Pathol. 1984, 81, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Lang, Y.; Yu, J.; Han, Z.; Chen, B.; Wang, Y. Affinity binding of aptamers to agarose with DNA tetrahedron for removal of hepatitis B virus surface antigen. Colloids Surf. B Biointerfaces 2019, 178, 80–86. [Google Scholar] [CrossRef]
- Shiesh, S.-C.; Ting, W.-K.; Jap, T.-S. Measurement of creatine kinase isoforms by agarose gel electrophoresis in the diagnosis of myocardial infarction. Clin. Biochem. 1992, 25, 293–301. [Google Scholar] [CrossRef]
- Stokols, S.; Sakamoto, J.; Breckon, C.; Holt, T.; Weiss, J.; Tuszynski, M.H. Templated agarose scaffolds support linear axonal regeneration. Tissue Eng. 2006, 12, 2777–2787. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, S.; Nieto, A.; Vallet-Regí, M. Hydroxyapatite/β-tricalcium phosphate/agarose macroporous scaffolds for bone tissue engineering. Chem. Eng. J. 2008, 137, 62–71. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive chitosan–agarose hydrogel for skin regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Pettikiriarachchi, J.T.; Parish, C.L.; Shoichet, M.S.; Forsythe, J.S.; Nisbet, D.R. Biomaterials for brain tissue engineering. Aust. J. Chem. 2010, 63, 1143–1154. [Google Scholar] [CrossRef]
- Troken, A.J.; Mao, J.J.; Marion, N.W.; Wan, L.Q.; Mow, V.C. Cartilage and Meniscus, Properties of. Encyclop. Med. Devices Instrum. 2006. [Google Scholar] [CrossRef]
- Hayami, T.; Pickarski, M.; Zhuo, Y.; Wesolowski, G.A.; Rodan, G.A.; Duong, L.T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 2006, 38, 234–243. [Google Scholar] [CrossRef]
- Setayeshmehr, M.; Esfandiari, E.; Rafieinia, M.; Hashemibeni, B.; Taheri-Kafrani, A.; Samadikuchaksaraei, A.; Kaplan, D.L.; Moroni, L.; Joghataei, M.T. Hybrid and composite scaffolds based on extracellular matrices for cartilage tissue engineering. Tissue Eng. Part B Rev. 2019, 25, 202–224. [Google Scholar] [CrossRef]
- Goldstein, A.S. Biomaterials for Cell Delivery: Vehicles in Regenerative Medicine; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Mahato, R.I. Biomaterials for Delivery and Targeting of Proteins and Nucleic Acids; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kwon, H.; Paschos, N.K.; Hu, J.C.; Athanasiou, K. Articular cartilage tissue engineering: The role of signaling molecules. Cell. Mol. Life Sci. 2016, 73, 1173–1194. [Google Scholar] [CrossRef] [Green Version]
- Usami, Y.; Gunawardena, A.T.; Iwamoto, M.; Enomoto-Iwamoto, M. Wnt signaling in cartilage development and diseases: Lessons from animal studies. Lab. Investig. 2015, 96, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Thorfve, A. Bone and Cartilage Regeneration: Wnt Signaling Pathway in Healing. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2014. [Google Scholar]
- Santos, G.; Doty, M. Agarose from Gracilaria cylindrica. Bot. Mar. 1983, 26, 31–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, X.; Duan, D.; Xu, J.; Gao, X. Preparation and characterization of agar, agarose, and agaropectin from the red alga Ahnfeltia plicata. J. Oceanol. Limnol. 2019, 37, 815–824. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Athukorala, Y.; Lee, J.-H. Characterization of agarose product from agar using DMSO. Algae 2005, 20, 61–67. [Google Scholar] [CrossRef]
- Din, S.S.; Chew, K.W.; Chang, Y.-K.; Show, P.L.; Phang, S.M.; Juan, J.C. Extraction of agar from Eucheuma cottonii and Gelidium amansii seaweeds with sonication pretreatment using autoclaving method. J. Oceanol. Limnol. 2019, 37, 871–880. [Google Scholar] [CrossRef]
- Kaupp, J.A.; Weber, J.F.; Waldman, S.D. Mechanical stimulation of chondrocyte-agarose hydrogels. J. Vis. Exp. 2012, 68, 4229. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, T.J.; Kumar, A. Efficient extraction of agarose from red algae using ionic liquids. Green Sustain. Chem. 2014, 4, 190. [Google Scholar] [CrossRef] [Green Version]
- Buskila, D.; Neumann, L.; Vaisberg, G.; Alkalay, D.; Wolfe, F. Increased rates of fibromyalgia following cervical spine injury. A controlled study of 161 cases of traumatic injury. Arthritis Rheum. 1997, 40, 446–452. [Google Scholar] [CrossRef]
- Singh, Y.P.; Bhardwaj, N.; Mandal, B.B. Potential of agarose/silk fibroin blended hydrogel for in vitro cartilage tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 21236–21249. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; D’Amora, U.; Ravalli, S.; Ambrosio, L.; Di Rosa, M.; Musumeci, G. Functional Biomolecule Delivery Systems and Bioengineering in Cartilage Regeneration. Curr. Pharm. Biotechnol. 2019, 20, 32–46. [Google Scholar] [CrossRef]
- Green, S. PEEK Biomaterials Handbook; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Anderson, J.M. Biocompatibility and bioresponse to biomaterials. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 675–694. [Google Scholar]
- Sefat, F.; Raja, T.I.; Zafar, M.S.; Khurshid, Z.; Najeeb, S.; Zohaib, S.; Ahmadi, E.D.; Rahmati, M.; Mozafari, M. Nanoengineered biomaterials for cartilage repair. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 39–71. [Google Scholar]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [Green Version]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Progress Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Kock, L.M.; Geraedts, J.; Ito, K.; Van Donkelaar, C.C. Low agarose concentration and TGF-β3 distribute extracellular matrix in tissue-engineered cartilage. Tissue Eng. Part A 2013, 19, 1621–1631. [Google Scholar] [CrossRef]
- Park, S.-J.; Goodman, M.B.; Pruitt, B.L. Measurement of mechanical properties of Caenorhabditis elegans with a piezoresistive microcantilever system. In Proceedings of the 3rd IEEE/EMBS Special Topic Conference on Microtechnology in Medicine and Biology, Oahu, HI, USA, 12–15 May 2005; pp. 400–403. [Google Scholar]
- Kandadai, M.A.; Raymond, J.L.; Shaw, G.J. Comparison of electrical conductivities of various brain phantom gels: Developing a ‘brain gel model’. Mater. Sci. Eng. C 2012, 32, 2664–2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scionti, G.; Moral, M.; Toledano, M.; Osorio, R.; Duran, J.D.; Alaminos, M.; Campos, A.; López, M.T. Effect of the hydration on the biomechanical properties in a fibrin-agarose tissue-like model. J. Biomed. Mater. Res. Part A 2014, 102, 2573–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S. Mechanical properties of biological materials. In Design of Artificial Human Joints & Organs; Springer: Berlin, Germany, 2014; pp. 23–40. [Google Scholar]
- Nguyen, B.V.; Wang, Q.G.; Kuiper, N.J.; El Haj, A.J.; Thomas, C.R.; Zhang, Z. Biomechanical properties of single chondrocytes and chondrons determined by micromanipulation and finite-element modelling. J. R. Soc. Interface 2010, 7, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Spedden, E.; White, J.D.; Naumova, E.N.; Kaplan, D.L.; Staii, C. Elasticity maps of living neurons measured by combined fluorescence and atomic force microscopy. Biophys. J. 2012, 103, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Ogneva, I.V.; Lebedev, D.V.; Shenkman, B.S. Transversal stiffness and Young’s modulus of single fibers from rat soleus muscle probed by atomic force microscopy. Biophys. J. 2010, 98, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Collinsworth, A.M.; Zhang, S.; Kraus, W.E.; Truskey, G.A. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am. J. Physiol. Cell Physiol. 2002, 283, C1219–C1227. [Google Scholar] [CrossRef] [Green Version]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [Green Version]
- Chen, E.J.; Novakofski, J.; Jenkins, W.K.; O’Brien, W.D. Young’s modulus measurements of soft tissues with application to elasticity imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1996, 43, 191–194. [Google Scholar] [CrossRef]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.; Wong, D.D.; Chao, P.-H.G.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.T.; Mauck, R.L.; Wang, C.C.-B.; Lima, E.G.; Ateshian, G.A. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann. Biomed. Eng. 2004, 32, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Roach, B.L.; Nover, A.B.; Ateshian, G.A.; Hung, C.T. Agarose hydrogel characterization for regenerative medicine applications: Focus on engineering cartilage. In Biomaterials from Nature for Advanced Devices and Therapies; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 258. [Google Scholar]
- Lee, H.-H.; Chang, C.-C.; Shieh, M.-J.; Wang, J.-P.; Chen, Y.-T.; Young, T.-H.; Hung, S.-C. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci. Rep. 2013, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guaccio, A.; Borselli, C.; Oliviero, O.; Netti, P. Oxygen consumption of chondrocytes in agarose and collagen gels: A comparative analysis. Biomaterials 2008, 29, 1484–1493. [Google Scholar] [CrossRef]
- Cigan, A.D.; Roach, B.L.; Nims, R.J.; Tan, A.R.; Albro, M.B.; Stoker, A.M.; Cook, J.L.; Vunjak-Novakovic, G.; Hung, C.T.; Ateshian, G.A. High seeding density of human chondrocytes in agarose produces tissue-engineered cartilage approaching native mechanical and biochemical properties. J. Biomech. 2016, 49, 1909–1917. [Google Scholar] [CrossRef] [Green Version]
- Selmi, T.A.S.; Verdonk, P.; Chambat, P.; Dubrana, F.; Potel, J.-F.; Barnouin, L.; Neyret, P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: Outcome at two years. J. Bone Jt. Surg. Br. Vol. 2008, 90, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Yao, H.; Huang, C.-Y.; Cheung, H.S. New insight into deformation-dependent hydraulic permeability of gels and cartilage, and dynamic behavior of agarose gels in confined compression. J. Biomech. 2003, 36, 593–598. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, X.; Jiang, Y.; Xing, L.; Xu, Y.; Lu, Y.; Ding, P.; Ma, J.; Xu, Y.; Gui, J. Platelet-rich plasma combined with agarose as a bioactive scaffold to enhance cartilage repair: An in vitro study. J. Biomater. Appl. 2014, 28, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Stilwell, M.D.; Santos, T.M.; Wang, H.; Weibel, D.B. Agarose particle-templated porous bacterial cellulose and its application in cartilage growth in vitro. Acta Biomater. 2015, 12, 129–138. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, B.J.; Dormer, N.H.; Ingavle, G.C.; Roatch, C.H.; Lomakin, J.; Detamore, M.S.; Gehrke, S.H. Hierarchically designed agarose and poly (ethylene glycol) interpenetrating network hydrogels for cartilage tissue engineering. Tissue Eng. Part C Methods 2010, 16, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sánchez-López, J.D.; Schaub, S.; Durán, J.D.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, Y.; Liu, B.; Cao, M.; Yang, J.; Tian, F.; Yang, P.; Qin, K.; Zhao, D. Basic fibroblast growth factor and agarose gel promote the ability of immune privilege of allogeneic cartilage transplantation in rats. J. Orthop. Transl. 2019. [Google Scholar] [CrossRef]





| Type of Signal | Role (Duty) |
|---|---|
| TGF-β | Regulates the proliferation/differentiation of chondrocytes, and stimulates the RY-box expression |
| BMP | Plays an important role in various stages of skeletal growth, and the commitment of mesenchymal cells to the lines of chondrocytes in the induction of proliferation and cell maturation in the growth and formation of the joints and bones |
| IGF | Develops cartilage and reproduces chondrocytes on the growth plate |
| FGF | Develops vital organs |
| Signaling Pathway | Role (Duty) |
|---|---|
| SMAD | Expresses pre-hypertrophic and proliferative and hypertrophic chondrocytes in all regions of the cartilage |
| b-catenin-dependent | Stimulates bone growth in axial growth, and induces endochondral ossification and axial growth |
| Non-canonical WNT | Creates growth pillars by chondrocytes |
| Material | Concentration % | Mechanical Properties (KPa) | Methods | Action | Ref. |
|---|---|---|---|---|---|
| Agarose | 1 | 10 | The effect of TGF-b3 was compared to the fatal bovine serum. Mechanical properties were assessed at day 42. | Low-concentration agarose stimulates the formation of more Homogeneous ECM distribution. The presence of TGF-b3 is also beneficial because it stimulates the distribution of matrix components. Both stimuli result in constructs with improved mechanical properties | [60] |
| Agarose | 2 | 16 | |||
| Agarose | 3 | 35 | |||
| Agarose-FBS | 1 | 15 | |||
| Agarose-FBS | 2 | 25 | |||
| Agarose-FBS | 3 | 40 | |||
| Agarose-TGF | 1 | 40 | |||
| Agarose-TGF | 2 | 45 | |||
| Agarose-TGF | 3 | 50 | |||
| Agarose-TGF | 0.25 | 40 | |||
| Agarose-TGF | 0.5 | 27 | |||
| Agarose-TGF | 1 | 34 | |||
| Agarose-TGF | 1 disc | 38 | |||
| Agarose | 2 | 1.547 | Gel | Both the volume fraction of water and hydraulic permeability decreased with increasing agarose gel concentration. Permeability was dependent on hydrogel and cartilage deformation | [78] |
| 2.5 | 4.237 | ||||
| 3 | 10.03 | ||||
| 4 | 10.55 | ||||
| 6 | 33.16 | ||||
| 10 | 82.34 | ||||
| 14.8 | 333.4 | ||||
| PRP-agarose | 7 days 14 days | 32 50 | Porcine chondrocytes were seeded in agarose gel and platelet-rich plasma–agarose gel. | The hydrogel possesses a proper microenvironment for chondrocyte growth, proliferation and matrix formation | [79] |
| 28 days | 75 | ||||
| AG-BM Strong modulus | 13.20 | Blended hydrogels | The degradation capability of blended hydrogel circumvents the drawbacks of the otherwise non-degradable pure agarose The hydrogels with non-mulberry SF blends showed larger pore size as compared to the mulberry blend hydrogels. The rheological studies revealed elasticity of the blended hydrogels had a yield point at a higher amplitude strain as compared to pure agarose | [53] | |
| AG-BM Loss modulus | 4.96 | ||||
| Controlled emulsion technique | [80] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. https://doi.org/10.3390/polym12051150
Salati MA, Khazai J, Tahmuri AM, Samadi A, Taghizadeh A, Taghizadeh M, Zarrintaj P, Ramsey JD, Habibzadeh S, Seidi F, et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers. 2020; 12(5):1150. https://doi.org/10.3390/polym12051150
Chicago/Turabian StyleSalati, Mohammad Amin, Javad Khazai, Amir Mohammad Tahmuri, Ali Samadi, Ali Taghizadeh, Mohsen Taghizadeh, Payam Zarrintaj, Josh D. Ramsey, Sajjad Habibzadeh, Farzad Seidi, and et al. 2020. "Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering" Polymers 12, no. 5: 1150. https://doi.org/10.3390/polym12051150
APA StyleSalati, M. A., Khazai, J., Tahmuri, A. M., Samadi, A., Taghizadeh, A., Taghizadeh, M., Zarrintaj, P., Ramsey, J. D., Habibzadeh, S., Seidi, F., Saeb, M. R., & Mozafari, M. (2020). Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers, 12(5), 1150. https://doi.org/10.3390/polym12051150