Effects of Solvent Vapor Annealing on Morphology and Charge Transport of Poly(3-hexylthiophene) (P3HT) Films Incorporated with Preformed P3HT Nanowires
Abstract
:1. Introduction
2. Materials and Methods
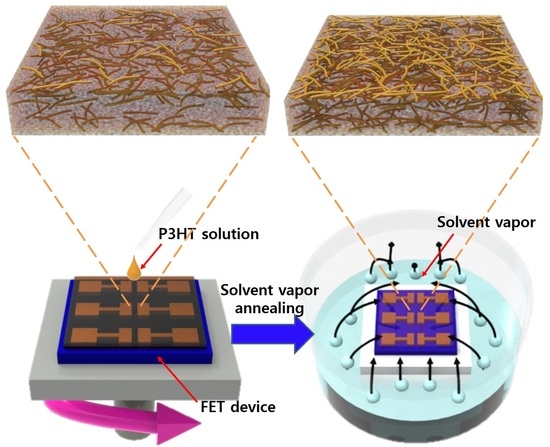
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grodd, L.; Mikayelyan, E.; Dane, T.; Pietsch, U.; Grigorian, S. Local scale structural changes of working OFET devices. Nanoscale 2020, 12, 2434–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandström, A.; Dam, H.F.; Krebs, F.C.; Edman, L. Ambient fabrication of flexible and large-area organic light-emitting devices using slot-die coating. Nat. Commun. 2012, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, S.Y.; Kim, Y.; Lee, J.; Lee, G.-Y.; Park, W.-T.; Noh, Y.-Y.; Park, C.E.; Park, T. High-field-effect mobility of low-crystallinity conjugated polymers with localized aggregates. J. Am. Chem. Soc. 2016, 138, 8096–8103. [Google Scholar] [CrossRef] [PubMed]
- Surya, S.G.; Raval, H.N.; Ahmad, R.; Sonar, P.; Salama, K.N.; Rao, V.R. Organic field effect transistors (OFETs) in environmental sensing and health monitoring: A review. TrAC Trends Anal. Chem. 2019, 111, 27–36. [Google Scholar] [CrossRef]
- Bjuggren, J.M.; Sharma, A.; Gedefaw, D.; Elmas, S.; Pan, C.; Kirk, B.; Zhao, X.; Andersson, G.; Andersson, M.R. Facile synthesis of an efficient and robust cathode interface material for polymer solar cells. ACS Appl. Energy Mater. 2018, 1, 7130–7139. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, P.; He, C.; Zhang, J.; Shen, P.; Zhang, Y.; Yi, Y.; Zhang, Z.; Li, Z.; Li, Y. Solution-processable organic molecule photovoltaic materials with bithienyl-benzodithiophene central unit and indenedione end groups. Chem. Mater. 2013, 25, 2274–2281. [Google Scholar] [CrossRef]
- Salleo, A. Charge transport in polymeric transistors. Mater. Today 2007, 10, 38–45. [Google Scholar] [CrossRef]
- Chang, M.; Choi, D.; Egap, E. Macroscopic alignment of one-dimensional conjugated polymer nanocrystallites for high-mobility organic field-effect transistors. ACS Appl. Mater. Interfaces 2016, 8, 13484–13491. [Google Scholar] [CrossRef]
- Luo, C.; Kyaw, A.K.K.; Perez, L.A.; Patel, S.; Wang, M.; Grimm, B.; Bazan, G.C.; Kramer, E.J.; Heeger, A.J. General strategy for self-assembly of highly oriented nanocrystalline semiconducting polymers with high mobility. Nano Lett. 2014, 14, 2764–2771. [Google Scholar] [CrossRef]
- Nikolka, M.; Hurhangee, M.; Sadhanala, A.; Chen, H.; McCulloch, I.; Sirringhaus, H. Correlation of disorder and charge transport in a range of indacenodithiophene-based semiconducting polymers. Adv. Electron. Mater. 2018, 4, 1700410. [Google Scholar] [CrossRef]
- Venkateshvaran, D.; Nikolka, M.; Sadhanala, A.; Lemaur, V.; Zelazny, M.; Kepa, M.; Hurhangee, M.; Kronemeijer, A.J.; Pecunia, V.; Nasrallah, I. Approaching disorder-free transport in high-mobility conjugated polymers. Nature 2014, 515, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Buckley, C.; Thomas, S.; McBride, M.; Yuan, Z.; Zhang, G.; Bredas, J.-L.; Reichmanis, E. Synergistic use of bithiazole and pyridinyl substitution for effective electron transport polymer materials. Chem. Mater. 2019, 31, 3957–3966. [Google Scholar] [CrossRef]
- Kim, B.-G.; Jeong, E.J.; Chung, J.W.; Seo, S.; Koo, B.; Kim, J. A molecular design principle of lyotropic liquid-crystalline conjugated polymers with directed alignment capability for plastic electronics. Nat. Mater. 2013, 12, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Hong, Z.; Li, G.; Yang, Y. Recent progress in polymer solar cells: Manipulation of polymer: Fullerene morphology and the formation of efficient inverted polymer solar cells. Adv. Mater. 2009, 21, 1434–1449. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, C.; Chang, J.; Yang, H.; Xi, H.; Chen, D.; Lin, Z.; Lu, G.; Zhang, J.; Hao, Y. Enhanced efficiency of planar perovskite solar cells via a two-step deposition using DMF as an additive to optimize the crystal growth behavior. J. Mater. Chem. A 2017, 5, 13032–13038. [Google Scholar] [CrossRef]
- Verploegen, E.; Mondal, R.; Bettinger, C.J.; Sok, S.; Toney, M.F.; Bao, Z. Effects of thermal annealing upon the morphology of polymer–fullerene blends. Adv. Funct. Mater. 2010, 20, 3519–3529. [Google Scholar] [CrossRef]
- Weller, T.; Breunig, M.; Müller, C.J.; Gann, E.; McNeill, C.; Thelakkat, M. Fluorination in thieno[3,4-c] pyrrole-4,6-dione copolymers leading to electron transport, high crystallinity and end-on alignment. J. Mater. Chem. C 2017, 5, 7527–7534. [Google Scholar] [CrossRef]
- Engmann, S.; Ro, H.W.; Herzing, A.; Snyder, C.R.; Richter, L.J.; Geraghty, P.B.; Jones, D.J. Film morphology evolution during solvent vapor annealing of highly efficient small molecule donor/acceptor blends. J. Mater. Chem. A 2016, 4, 15511–15521. [Google Scholar] [CrossRef]
- Khim, D.; Baeg, K.-J.; Kim, J.; Kang, M.; Lee, S.-H.; Chen, Z.; Facchetti, A.; Kim, D.-Y.; Noh, Y.-Y. High performance and stable N-channel organic field-effect transistors by patterned solvent-vapor annealing. ACS Appl. Mater. Interfaces 2013, 5, 10745–10752. [Google Scholar] [CrossRef]
- Chang, M.; Lim, G.T.; Park, B.; Reichmanis, E. Control of molecular ordering, alignment, and charge transport in solution-processed conjugated polymer thin films. Polymers 2017, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Jo, G.; Jung, J.; Chang, M. Controlled self-assembly of conjugated polymers via a solvent vapor pre-treatment for use in organic field-effect transistors. Polymers 2019, 11, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.-K.; Shin, E.-S.; Noh, Y.-Y.; Kim, D.-Y. A selection rule of solvent for highly aligned diketopyrrolopyrrole-based conjugated polymer film for high performance organic field-effect transistors. Org. Electron. 2018, 55, 6–14. [Google Scholar] [CrossRef]
- Chang, M.; Lee, J.; Chu, P.-H.; Choi, D.; Park, B.; Reichmanis, E. Anisotropic assembly of conjugated polymer nanocrystallites for enhanced charge transport. ACS Appl. Mater. Interfaces 2014, 6, 21541–21549. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Lee, J.; Kleinhenz, N.; Fu, B.; Reichmanis, E. Photoinduced anisotropic supramolecular assembly and enhanced charge transport of poly(3-hexylthiophene) thin films. Adv. Funct. Mater. 2014, 24, 4457–4465. [Google Scholar] [CrossRef]
- Chang, M.; Su, Z.; Egap, E. Alignment and charge transport of one-dimensional conjugated polymer nanowires in insulating polymer blends. Macromolecules 2016, 49, 9449–9456. [Google Scholar] [CrossRef]
- Charvet, R.; Ariga, K.; Hill, J.P.; Ji, Q.; Khan, A.H.; Acharya, S. Large scale assembly of ordered donor–acceptor heterojunction molecular wires using the Langmuir–Blodgett technique. Chem. Commun. 2011, 47, 6825–6827. [Google Scholar] [CrossRef]
- Khim, D.; Han, H.; Baeg, K.J.; Kim, J.; Kwak, S.W.; Kim, D.Y.; Noh, Y.Y. Simple bar-coating process for large-area, high-performance organic field-effect transistors and ambipolar complementary integrated circuits. Adv. Mater. 2013, 25, 4302–4308. [Google Scholar] [CrossRef]
- Kotsuki, K.; Obata, S.; Saiki, K. Self-aligned growth of organic semiconductor single crystals by electric field. Langmuir 2016, 32, 644–649. [Google Scholar] [CrossRef]
- McFarland, F.M.; Liu, X.; Zhang, S.; Tang, K.; Kreis, N.K.; Gu, X.; Guo, S. Electric field induced assembly of macroscopic fibers of poly(3-hexylthiophene). Polymer 2018, 151, 56–64. [Google Scholar] [CrossRef]
- Persson, N.E.; Chu, P.-H.; McBride, M.; Grover, M.; Reichmanis, E. Nucleation, growth, and alignment of poly(3-hexylthiophene) nanofibers for high-performance OFETs. Acc. Chem. Res. 2017, 50, 932–942. [Google Scholar] [CrossRef]
- Dickey, K.C.; Anthony, J.E.; Loo, Y.L. Improving organic thin-film transistor performance through solvent-vapor annealing of solution-processable triethylsilylethynyl anthradithiophene. Adv. Mater. 2006, 18, 1721–1726. [Google Scholar] [CrossRef]
- Mascaro, D.J.; Thompson, M.E.; Smith, H.I.; Bulović, V. Forming oriented organic crystals from amorphous thin films on patterned substrates via solvent-vapor annealing. Org. Electron. 2005, 6, 211–220. [Google Scholar] [CrossRef]
- Tang, H.; Lu, G.; Li, L.; Li, J.; Wang, Y.; Yang, X. Precise construction of PCBM aggregates for polymer solar cells via multi-step controlled solvent vapor annealing. J. Mater. Chem. 2010, 20, 683–688. [Google Scholar] [CrossRef]
- Wessendorf, C.D.; Schulz, G.L.; Mishra, A.; Kar, P.; Ata, I.; Weidelener, M.; Urdanpilleta, M.; Hanisch, J.; Mena-Osteritz, E.; Lindén, M. Efficiency improvement of solution-processed dithienopyrrole-based A-D-A oligothiophene bulk-heterojunction solar cells by solvent vapor annealing. Adv. Energy Mater. 2014, 4, 1400266. [Google Scholar] [CrossRef]
- Jeong, J.W.; Jo, G.; Choi, S.; Kim, Y.A.; Yoon, H.; Ryu, S.-W.; Jung, J.; Chang, M. Solvent additive-assisted anisotropic assembly and enhanced charge transport of π-conjugated polymer thin films. ACS Appl. Mater. Interfaces 2018, 10, 18131–18140. [Google Scholar] [CrossRef]
- Holliday, S.; Ashraf, R.S.; Wadsworth, A.; Baran, D.; Yousaf, S.A.; Nielsen, C.B.; Tan, C.-H.; Dimitrov, S.D.; Shang, Z.; Gasparini, N. High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 2016, 7, 11585. [Google Scholar] [CrossRef] [Green Version]
- Ikawa, M.; Yamada, T.; Matsui, H.; Minemawari, H.; Tsutsumi, J.y.; Horii, Y.; Chikamatsu, M.; Azumi, R.; Kumai, R.; Hasegawa, T. Simple push coating of polymer thin-film transistors. Nat. Commun. 2012, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef]
- Park, Y.D.; Lee, H.S.; Choi, Y.J.; Kwak, D.; Cho, J.H.; Lee, S.; Cho, K. Solubility-induced ordered polythiophene precursors for high-performance organic thin-film transistors. Adv. Funct. Mater. 2009, 19, 1200–1206. [Google Scholar] [CrossRef]
- Zhao, K.; Khan, H.U.; Li, R.; Su, Y.; Amassian, A. Entanglement of conjugated polymer chains influences molecular self-assembly and carrier transport. Adv. Funct. Mater. 2013, 23, 6024–6035. [Google Scholar] [CrossRef]
- Bhatt, M.; Magurudeniya, H.; Rainbolt, E.; Huang, P.; Dissanayake, D.; Biewer, M.; Stefan, M. Poly(3-hexylthiophene) nanostructured materials for organic electronics applications. J. Nanosci. Nanotechnol. 2014, 14, 1033–1050. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Razzano, V.; Paolino, M.; Grisci, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Samperi, F.; Battiato, S.; Boccia, A.C. Bithiophene-based polybenzofulvene derivatives with high stacking and hole mobility. Polym. Chem. 2015, 6, 7377–7388. [Google Scholar] [CrossRef]
- McFarland, F.M.; Bonnette, L.R.; Acres, E.A.; Guo, S. The impact of aggregation on the p-doping kinetics of poly(3-hexylthiophene). J. Mater. Chem. C 2017, 5, 5764–5771. [Google Scholar] [CrossRef] [PubMed]
- Hildner, R.; Köhler, A.; Müller-Buschbaum, P.; Panzer, F.; Thelakkat, M. π-conjugated donor polymers: Structure formation and morphology in solution, bulk and photovoltaic blends. Adv. Energy Mater. 2017, 7, 1700314. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.; Chang, J.-F.; Spano, F.C.; Friend, R.H.; Silva, C. Determining exciton bandwidth and film microstructure in polythiophene films using linear absorption spectroscopy. Appl. Phys. Lett. 2009, 94, 117. [Google Scholar] [CrossRef]
- Chang, M.; Choi, D.; Fu, B.; Reichmanis, E. Solvent based hydrogen bonding: Impact on poly(3-hexylthiophene) nanoscale morphology and charge transport characteristics. ACS Nano 2013, 7, 5402–5413. [Google Scholar] [CrossRef]
- Liu, J.; Haynes, D.; Balliet, C.; Zhang, R.; Kowalewski, T.; McCullough, R.D. Self encapsulated poly(3-hexylthiophene)-poly(fluorinated alkyl methacrylate) rod-coil block copolymers with high field effect mobilities on bare SiO2. Adv. Funct. Mater. 2012, 22, 1024–1032. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, M.; Huh, Y.-I.; Chang, M. Effects of Solvent Vapor Annealing on Morphology and Charge Transport of Poly(3-hexylthiophene) (P3HT) Films Incorporated with Preformed P3HT Nanowires. Polymers 2020, 12, 1188. https://doi.org/10.3390/polym12051188
Jang M, Huh Y-I, Chang M. Effects of Solvent Vapor Annealing on Morphology and Charge Transport of Poly(3-hexylthiophene) (P3HT) Films Incorporated with Preformed P3HT Nanowires. Polymers. 2020; 12(5):1188. https://doi.org/10.3390/polym12051188
Chicago/Turabian StyleJang, Mingu, Yang-Il Huh, and Mincheol Chang. 2020. "Effects of Solvent Vapor Annealing on Morphology and Charge Transport of Poly(3-hexylthiophene) (P3HT) Films Incorporated with Preformed P3HT Nanowires" Polymers 12, no. 5: 1188. https://doi.org/10.3390/polym12051188
APA StyleJang, M., Huh, Y. -I., & Chang, M. (2020). Effects of Solvent Vapor Annealing on Morphology and Charge Transport of Poly(3-hexylthiophene) (P3HT) Films Incorporated with Preformed P3HT Nanowires. Polymers, 12(5), 1188. https://doi.org/10.3390/polym12051188