A Study of the Reinforcement Effect of MWCNTs onto Polyimide Flat Sheet Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of MWCNTs/Polyimide Nanocomposite Films
2.3. Characterization Techniques
3. Results
3.1. Morphology of MWCNTs and Nanocomposite Materials
3.2. Thermal Analysis
3.3. FTIR Analysis
3.4. Mechanical Properties of MWCNTs/Polyimide Nanocomposite Films
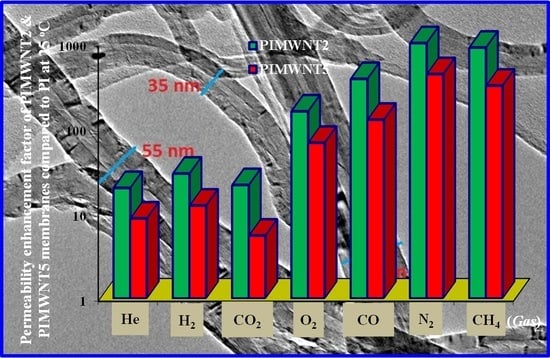
3.5. Gas Permeability Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons Ltd.: Chichester, England, 2004. [Google Scholar]
- Hamid, M.R.A.; Jeong, H.-K. Recent advances on mixed-matrix membranes for gas separation: Opportunities and engineering challenges. Korean J. Chem. Eng. 2018, 35, 1577–1600. [Google Scholar] [CrossRef]
- Perdikaki, A.V.; Labropoulos, A.I.; Siranidi, E.; Karatasios, I.; Kanellopoulos, N.; Boukos, N.; Falaras, P.; Karanikolos, G.N.; Romanos, G.E. Efficient CO oxidation in an ionic liquid-modified, Au nanoparticle-loaded membrane contactor. Chem. Eng. J. 2016, 305, 79–91. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous Inorganic Membranes for CO2 Capture: Present and Prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef]
- Alomair, A.A.; Al-Jubouri, S.M.; Holmes, S.M. A novel approach to fabricate zeolite membranes for pervaporation processes. J. Mater. Chem. A 2015, 3, 9799–9806. [Google Scholar] [CrossRef]
- Nasir, R.; Mukhtar, H.; Man, Z.; Mohshim, D.F. Material Advancements in Fabrication of Mixed-Matrix Membranes. Chem. Eng. Technol. 2013, 36, 717–727. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Dong, H.; Meng, M.; Li, C.; Yan, Y.; Chen, J. An overview on membrane strategies for rare earths extraction and separation. Sep. Purif. Technol. 2018, 197, 70–85. [Google Scholar] [CrossRef]
- Sunarso, J.; Hashim, S.S.; Lin, Y.S.; Liu, S.M. Membranes for helium recovery: An overview on the context, materialsand future directions. Sep. Purif. Technol. 2017, 176, 335–383. [Google Scholar] [CrossRef]
- Favvas, E.P.; Heliopoulos, N.S.; Devlin, E.; Karousos, D.S.; Papageorgiou, S.K.; Petridis, D.; Karanikolos, G.N. Mixed Matrix Polymeric and Carbon Hollow Fiber Membranes with Magnetic Iron-Based Nanoparticles and their Application in Gas Mixture Separation. Mater. Chem. Phys. 2019, 223, 220–229. [Google Scholar] [CrossRef]
- Ji, Y.; Qian, W.; Yu, Y.; An, Q.; Liu, L.; Zhou, Y.; Gao, C. Recent developments in nanofiltration membranes based on nanomaterials. Chin. J. Chem. Eng. 2017, 25, 1639–1652. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Balta, S.; Kimd, J.; der Bruggen, B.V. Doping of polyethersulfone nanofiltration membranes: Antifouling effect observed at ultralow concentrations of TiO2 nanoparticles. J. Mater. Chem. 2011, 21, 10311–10320. [Google Scholar] [CrossRef]
- Eremin, Y.S.; Bakhtin, D.; Pavlov, S.V.; Grekhov, A.M. Effect of carbon nanotubes on a gases permeability of polymer PMMA. J. Phys. Conf. Ser. 2018, 1099, 012036. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Norouzi, M.; Zare, Y.; Kiany, P. Nanoparticles as Effective Flame Retardants for Natural and Synthetic Textile Polymers: Application, Mechanism, and Optimization. Polym. Rev. 2015, 55, 531–560. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Progr. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, L. Polymer Nanocomposites for Electrical Energy Storage. J. Polym. Sci. Pol. Phys. 2011, 49, 1421–1429. [Google Scholar] [CrossRef]
- Yang, C.; Wei, H.; Guan, L.; Guo, J.; Wang, Y.; Yan, X.; Zhang, X.; Wei, S.; Guo, Z. Polymer nanocomposites for energy storage, energy saving, and anticorrosion. J. Mater. Chem. A 2015, 3, 14929–14941. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zhang, Z.; Hao, Z. Mesoporous KIT-6 silica–polydimethylsiloxane (PDMS) mixed matrix membranes for gas separation. J. Mater. Chem. A 2015, 3, 8650–8658. [Google Scholar] [CrossRef]
- Zarshenas, K.; Raisi, A.; Aroujalian, A. Mixed matrix membrane of nano-zeolite NaX/poly (ether-block-amide) for gas separation applications. J. Membr. Sci. 2016, 510, 270–283. [Google Scholar] [CrossRef]
- Favvas, E.P.; Nitodas, S.F.; Stefopoulos, A.; Stefanopoulos, K.L.; Papageorgiou, S.K.; Mitropoulos, A.C. High Purity Multi-Walled Carbon Nanotubes: Preparation, Characterization and Performance as Filler Materials in co-polyimide Hollow Fiber Membranes. Sep. Purif. Technol. 2014, 122, 262–269. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Peng, K.-J.; Liu, Y.-L.; Deng, L. Pebax/PEG Grafted CNT Hybrid Membranes for Enhanced CO2/N2 Separation. Ind. Eng. Chem. Res. 2019, 58, 12226–12234. [Google Scholar] [CrossRef]
- Melicchio, A.; Favvas, E.P. Preparation and characterization of graphene oxide as a candidate filler material for the preparation of mixed matrix polyimide membranes. Surf. Coat. Technol. 2018, 349, 1058–1068. [Google Scholar] [CrossRef]
- He, R.; Cong, S.; Wang, J.; Liu, J.; Zhang, Y. Porous Graphene Oxide/Porous Organic Polymer Hybrid Nanosheets Functionalized Mixed Matrix Membrane for Efficient CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 4338–4344. [Google Scholar] [CrossRef] [PubMed]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X.; Gascon, J. Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2014, 14, 48–55. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Zhai, L.; Liu, G.; Dong, J.; Wang, Y.; Christopher, M.P.; Long, S.; Wang, Y.; Zhao, D. Mixed matrix membranes containing MOF@COF hybrid fillers for efficient CO2/CH4 separation. J. Membr. Sci. 2019, 573, 97–106. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Narh, K.A.; Jallo, L.; Rhee, K.Y. The Effect of Carbon Nanotube Agglomeration on the Thermal and Mechanical Properties of Polyethylene Oxide. Polym. Comp. 2008, 29, 809–817. [Google Scholar] [CrossRef]
- Zhbanov, A.I.; Pogorelov, E.G.; Chang, Y.-C. Van der Waals interaction between two crossed carbon nanotubes. ACS Nano 2010, 4, 5937–5945. [Google Scholar] [CrossRef] [Green Version]
- Favvas, E.P.; Stefanopoulos, K.L.; Nolan, J.W.; Papageorgiou, S.K.; Mitropoulos, A.C.; Lairez, D. Mixed Matrix Hollow Fiber Membranes with enhanced gas permeation properties. Sep. Purif. Technol. 2014, 132, 336–345. [Google Scholar] [CrossRef]
- Dustebek, J.; Kandemir-Cavas, C.; Nitodas, S.F.; Cavas, L. Effects of carbon nanotubes on the mechanical strength of self-polishing antifouling paints. Prog. Org. Coat. 2016, 98, 18–27. [Google Scholar] [CrossRef]
- Favvas, E.P.; Stefanopoulos, K.L.; Stefopoulos, A.A.; Nitodas, S.F.; Mitropoulos, A.C.; Lairez, D. Phenol functionalized MWCNTs: A dispersion study into polar solvents by Small Angle Neutron Scattering. Colloids Surf. A 2016, 496, 94–99. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Favvas, E.P.; Katsaros, F.K.; Papageorgiou, S.K.; Sapalidis, A.A.; Mitropoulos, A.C. A Review of the Latest Development of Polyimide based Membranes for CO2 Separations. React. Funct. Polym. 2017, 120, 104–130. [Google Scholar] [CrossRef]
- Burc, M.; Koytepe, S.; Duran, S.T.; Ayhan, N.; Aksoy, B.; Seckin, T. Development of voltammetric sensor based on polyimide-MWCNT composite membrane for rapid and highly sensitive detection of paracetamol. Measurement 2020, 151, 107103. [Google Scholar] [CrossRef]
- Shigeta, M.; Komatsu, M.; Nakashima, N. Individual solubilization of single-walled carbon nanotubes using totally aromatic polyimide. Chem. Phys. Lett. 2006, 418, 115–118. [Google Scholar] [CrossRef]
- Smith, J.G.; Connell, J.W.; Delozier, D.M.; Lillehei, P.T.; Watson, K.A.; Lin, Y.; Zhou, B.; Sun, Y.P. Space durable polymer/carbon nanotube films for electrostatic charge mitigation. Polymer 2004, 45, 825–836. [Google Scholar] [CrossRef]
- Smith, J.G.; Delozier, D.M.; Connell, J.W.; Watson, K.A. Carbon nanotube–conductive additive-space durable polymer nanocomposite films for electrostatic charge dissipation. Polymer 2004, 45, 6133–6142. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.; Lin, Y.; Qu, L.; Kitaygorodskiy, A.; Connell, J.W.; Allard, L.F.; Sun, Y.P. Functionalization of carbon nanotubes with derivatized polyimide. Macromolecules 2005, 38, 7670–7675. [Google Scholar] [CrossRef]
- Ibrahim, N.N.I.N.; Romli, A.Z. Mechanical properties of one layer and seven layer glass fibre reinforced unsaturated polyester filled with P84 polyimide. In AIP Conference Proceedings; AIP Publishing LLC: College Park, MD, USA, 2018; Volume 1985, p. 030008. [Google Scholar]
- Cavas, L.; Gokfuliz, P.; Mimigianni, P.; Sapalidis, A.; Nitodas, S. Reinforcement effects of multiwall carbon nanotubes and graphene oxide on PDMS marine coatings. J. Coat. Technol. Res. 2018, 15, 105–120. [Google Scholar] [CrossRef]
- Park, C.H.; Tocci, E.; Fontananova, E.; Bahattab, M.A.; Aljlil, S.A.; Drioli, E. Mixed matrix membranes containing functionalized multiwalled carbon nanotubes: Mesoscale simulation and experimental approach for optimizing dispersion. J. Membr. Sci. 2016, 514, 195–209. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kouvelos, E.P.; Romanos, G.E.; Pilatos, G.Ι.; Mitropoulos, A.C.; Kanellopoulos, N.K. Characterization of highly selective microporous carbon hollow fiber membranes prepared from a commercial co-polyimide precursor. J. Porous Mater. 2008, 15, 625–633. [Google Scholar] [CrossRef]
- Sapalidis, A.A.; Katsaros, F.K.; Romanos, G.E.; Kakizis, N.K.; Kanellopoulos, N.K. Preparation and characterization of novel poly-(vinyl alcohol)–Zostera flakes composites for packaging applications. Compos. Part B 2007, 38, 398–404. [Google Scholar] [CrossRef]
- Sapalidis, A.A.; Katsaros, F.K.; Steriotis, T.A.; Kanellopoulos, N.K. Properties of Poly(vinyl alcohol)—Bentonite Clay Nanocomposite Films in Relation to Polymer–Clay Interactions. J. Appl. Polym. Sci. 2012, 123, 1812–1821. [Google Scholar] [CrossRef]
- Qiao, X.; Chung, T.-S. Diamine modification of P84 polyimide membranes for pervaporation dehydration of isopropanol. AIChE J. 2006, 52, 3462–3472. [Google Scholar] [CrossRef]
- Kuan, C.F.; Chen, W.J.; Li, Y.L.; Chen, C.H.; Kuan, H.C.; Chiang, C.L. Flame retardance and thermal stability of carbon nanotube epoxy composite prepared from sol–gel method. J. Phys. Chem. Solids 2010, 71, 539–543. [Google Scholar] [CrossRef]
- Ismail, A.F.; Kusworo, T.D.; Mustafa, A. Enhanced gas permeation performance of polyethersulfone mixed matrix hollow fiber membranes using novel Dynasylan Ameo silane agent. J. Membr. Sci. 2008, 319, 306–312. [Google Scholar] [CrossRef]
- Mangindaan, D.W.; Woon, N.M.; Shi, G.M.; Chung, T.S. P84 polyimide membranes modified by a tripodal amine for enhanced pervaporation dehydration of acetone. Chem. Eng. Sci. 2015, 122, 14–23. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, C.-C.; Xu, L.; Cui, L.; Paul, D.R.; Koros, W.J. Sub-Tg Cross-Linking of a Polyimide Membrane for Enhanced CO2 Plasticization Resistance for Natural Gas Separation. Macromolecules 2011, 44, 6046–6056. [Google Scholar] [CrossRef]
- Maya, E.M.; Tena, A.; de Abajo, J.; de la Campa, J.G.; Lozano, A.E. Partially pyrolyzed membranes (PPMs) derived from copolyimides having carboxylic acid groups. Prep. Gas Transp. Prop. J. Membr. Sci. 2010, 349, 385–392. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Sazali, N.; Ismail, A.F. Development and characterization of disk supported carbon membrane prepared by one-step coating-carbonization cycle. J. Industr. Eng. Chem. 2018, 57, 313–321. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Structural and transport properties of BTDA-TDI/MDI co-polyimide (P84)–silica nanocomposite membranes for gas separation. Chem. Eng. J. 2012, 188, 199–209. [Google Scholar] [CrossRef]
- Alexopoulos, N.D.; Gegitsidis, F.D.; Kourkoulis, S.K.; Favvas, E.P. Mechanical behavior of MWCNTs based mixed-matrix polymeric and carbon hollow fiber membranes. Sep. Purif. Technol. 2017, 183, 21–31. [Google Scholar] [CrossRef]
- Salleh, W.N.W.; Ismail, A.F. Effect of Stabilization Temperature on Gas Permeation Properties of Carbon Hollow Fiber Membrane. J. Appl. Polym. Sci. 2013, 127, 2840–2846. [Google Scholar] [CrossRef]
- Le, N.L.; Chung, T.S. High-performance sulfonated polyimide/polyimide/polyhedral oligosilsesquioxane hybrid membranes for ethanol dehydration applications. J. Membr. Sci. 2014, 454, 62–73. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Wang, Y.; Chung, T.S.; Qiao, X.Y.; Lai, J.Y. Polyimides membranes for pervaporation and biofuels separation. Prog. Polym. Sci. 2009, 34, 1135–1160. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Effects of Membrane Thickness and Heat Treatment on the Gas Transport Properties of Membranes Based on P84 Polyimide. J. Appl. Polym. Sci. 2010, 116, 2906–2912. [Google Scholar] [CrossRef]
- Barsema, J.N.; Kapantaidakis, G.C.; van der Vegt, N.F.A.; Koops, G.H.; Wessling, M. Preparation and characterization of highly selective dense and hollow fiber asymmetric membranes based on BTDA-TDI/MDI co-polyimide. J. Membr. Sci. 2003, 216, 195–205. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Riccardo, R.; Simone, L.; Meganne, C.; Vittorio, M.; Marco, G.B.; Maria, G.D.A. Permeability and Selectivity of PPO/Graphene Composites as Mixed Matrix Membranes for CO2 Capture and Gas Separation. Polymers 2018, 10, 129. [Google Scholar]
- Wong, K.C.; Goh, P.S.; Ismail, A.F. Carbon-Based Nanocomposite Membrane for Acidic Gas Separation. In Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications; Ismail, A.F., Goh, P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 233–260. [Google Scholar]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Wang, C.; Chang, H.-C.; Guo, R. Carbon nanotube-based mixed-matrix membranes with supramolecularly engineered interface for enhanced gas separation performance. J. Membr. Sci. 2020, 598, 117794. [Google Scholar] [CrossRef]
- Wang, D.; Yao, D.; Wang, Y.; Wang, F.; Xin, Y.; Song, S.; Zhang, Z.; Su, F.; Zheng, Y. Carbon nanotubes and graphene oxide-based solvent-free hybrid nanofluids functionalized mixed-matrix membranes for efficient CO2/N2 separation. Sep. Purif. Technol. 2019, 221, 421–432. [Google Scholar] [CrossRef]
- Modi, A.; Verma, S.K.; Bellare, J. Carboxylated Carbon Nanotubes/Polyethersulfone Hollow Fiber Mixed Matrix Membranes: Development and Characterization for Enhanced Gas Separation Performance. MRS Adv. 2018, 3, 3103. [Google Scholar] [CrossRef]
- Habibiannejad, S.A.; Aroujalian, A.; Raisi, A. Pebax-1657 mixed matrix membrane containing surface modified multi-walled carbon nanotubes for gas separation. RSC Adv. 2016, 6, 79563. [Google Scholar] [CrossRef]
- Borgohain, R.; Jain, N.; Prasad, B.; Mandal, B.; Su, B. Carboxymethyl chitosan/carbon nanotubes mixed matrix membranes for CO2 separation. React. Funct. Polym. 2019, 14, 104331. [Google Scholar] [CrossRef]
- Songa, Y.; Xu, L. Permeability, thermal and wetting properties of aligned composite nanofiber membranes containing carbon nanotubes. Int. J. Hydrogen Energy 2017, 42, 19961–19966. [Google Scholar] [CrossRef]
- Swain, S.S.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Carbon nanotubes as potential candidate for separation of H2/CO2 gas pairs. Int. J. Hydrogen Energy 2017, 42, 29283–29299. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, T.; Liu, Z.; Su, Y.; Yu, H.; Ma, J.; Yang, Y.; Jiang, Z. Synthesis and properties of sulfonated biphenyl poly(ether sulfone) and its mixed-matrix membranes containing carbon nanotubes for gas separation. J. Appl. Polym. Sci. 2017, 134, 44995. [Google Scholar] [CrossRef]
- Vengatesan, M.; Wahab, M.A.; Kuppireddy, S.; Kakosimos, G.; Abdalla, O.; Favvas, E.; Reinalda, D.; Geuzebroek, F.; Abdala, A.; Karanikolos, G. Metal-Organic Framework-Based Mixed Matrix Membranes for Carbon Dioxide Separation: Recent Advances and Future Directions. Front. Chem. 2020. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Montazer-Rahmati, M.M.; Matsuura, T. Effect of chitosan as a functionalization agent on the performance and separation properties of polyimide/multi-walled carbon nanotubes mixed matrix flat sheet membranes. J. Membr. Sci. 2010, 364, 309–317. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Matsuura, T. Beta-cyclodextrin functionalized MWCNT: A potential nano-membrane material for mixed matrix gas separation membranes development. Sep. Purif. Technol. 2013, 115, 39–50. [Google Scholar] [CrossRef]
- Bighane, N.; Koros, W.J. Novel silica membranes for high temperature gas separations. J. Membr. Sci. 2011, 371, 254–262. [Google Scholar] [CrossRef]
- Xianshe, F.; Huang, R.Y.M. Estimation of activation energy for permeation in pervaporation Processes. J. Membr. Sci. 1996, 118, 127–131. [Google Scholar] [CrossRef]
- Robeson, L.M.; Burgoyne, W.F.; Langsam, M.; Savoca, A.C.; Tien, C.F. High performance polymers for membrane separation. Polymer 1994, 35, 4970–4978. [Google Scholar] [CrossRef]
- Escorihuela, S.; Tena, A.; Shishatskiy, S.; Escolástico, S.; Brinkmann, T.; Serra, J.M.; Abetz, V. Gas Separation Properties of Polyimide Thin Films on Ceramic Supports for High Temperature Applications. Membranes 2018, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Dixon-Garrett, S.V.; Nagai, K.; Freeman, B.D. Ethylbenzene Solubility, Diffusivity, and Permeability in Poly(dimethylsiloxane). J. Polym. Sci. Pol. Phys. 2000, 38, 1461–1473. [Google Scholar] [CrossRef]
- Bao, L.; Dorgan, J.R.; Knauss, D.; Hait, S.; Oliveira, N.S.; Maruccho, I.M. Gas permeation properties of poly(lactic acid) revisited. J. Membr. Sci. 2006, 285, 166–172. [Google Scholar] [CrossRef]
- Pinnau, I.; He, Z. Pure- and mixed-gas permeation properties of polydimethylsiloxane for hydrocarbon/methane and hydrocarbon/hydrogen separation. J. Membr. Sci. 2004, 244, 227–233. [Google Scholar] [CrossRef]












| Sample Code | Polyimide | MWCNTs |
|---|---|---|
| wt.% | wt.% | |
| PI | 100 | 0 |
| PIMWNT1 | 99 | 1 |
| PIMWNT2 | 98 | 2 |
| PIMWNT3 | 97 | 3 |
| PIMWNT5 | 95 | 5 |
| Membrane | PI | PIMWNT1 | PIMWNT2 | PIMWNT3 | PIMWNT5 |
|---|---|---|---|---|---|
| Mass Loss (%) at 900 °C | 40.5 | 38.2 | 40.5 | 42 | 39.5 |
| Sample | Young’s Modulus GPa | Elongation % | Tensile Strength MPa |
|---|---|---|---|
| PI | 1.373 ± 0.09 | 3.98 ± 0.77 | 45.8 ± 7.50 |
| PIMWNT1 | 1.544 ± 0.16 | 4.85 ± 0.17 | 65.1 ± 5.41 |
| PIMWNT2 | 1.653 ± 0.18 | 5.12 ± 0.94 | 73.6 ± 6.11 |
| PIMWNT3 | 1.487 ± 0.09 | 4.17 ± 0.56 | 55.8 ± 2.52 |
| PIMWNT5 | 1.173 ± 0.08 | 3.07 ± 0.42 | 24.7 ± 3.61 |
| Relative Humidity (%) | Permeability (Barrer) | ||||
|---|---|---|---|---|---|
| Membrane | |||||
| PI | PIMWNT1 | PIMWNT2 | PIMWNT3 | PIMWNT5 | |
| 0 | 0.60 ± 0.014 | 0.87 ± 0.023 | 1.18 ± 0.027 | 0.90 ± 0.022 | 0.72 ± 0.030 |
| 25 | 0.40 ± 0.008 | 0.59 ± 0.017 | 0.78 ± 0.023 | 0.57 ± 0.015 | 0.49 ± 0.009 |
| 50 | 0.31 ± 0.009 | 0.42 ± 0.008 | 0.61 ± 0.024 | 0.42 ± 0.011 | 0.35 ± 0.007 |
| 65 | 0.27 ± 0.007 | 0.36 ± 0.009 | 0.55 ± 0.017 | 0.40 ± 0.098 | 0.29 ± 0.007 |
| 75 | 0.26 ± 0.010 | 0.34 ± 0.008 | 0.53 ± 0.015 | 0.38 ± 0.011 | 0.29 ± 0.006 |
| 85 | 0.28 ± 0.007 | 0.34 ± 0.009 | 0.53 ± 0.014 | 0.38 ± 0.010 | 0.28 ± 0.007 |
| GAS | Permeability (Barrer) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 °C | 50 °C | 100 °C | |||||||
| PI | PIMWNT2 | PIMWNT5 | PI | PIMWNT2 | PIMWNT5 | PI | PIMWNT2 | PIMWNT5 | |
| He | 9.6 | 189.8 | 83.2 | 10.6 | 112.3 | 69.5 | 40.3 | 103.1 | 81.3 |
| H2 | 9.9 | 289 | 121.7 | 15.9 | 261.7 | 101.1 | 41 | 255.6 | 119.8 |
| CO2 | 8.9 | 190.5 | 48.5 | 10.2 | 50.5 | 38.2 | 17.4 | 46.3 | 35.8 |
| O2 | 0.6 | 94.5 | 40.4 | 1.6 | 44.9 | 29.6 | 4.5 | 41.2 | 27.4 |
| CO | 0.3 | 113.8 | 37.7 | 0.5 | 52.3 | 28.9 | 2.1 | 49.6 | 32.9 |
| N2 | 0.1 | 105.4 | 43.2 | 0.3 | 47.4 | 30.1 | 1.7 | 43.6 | 24.8 |
| CH4 | 0.2 | 177.3 | 63.3 | 0.6 | 75.6 | 48.7 | 2.9 | 60.8 | 51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapalidis, A.A.; Karantzis, P.I.; Vairis, A.; Nitodas, S.F.; Barbe, S.; Favvas, E.P. A Study of the Reinforcement Effect of MWCNTs onto Polyimide Flat Sheet Membranes. Polymers 2020, 12, 1381. https://doi.org/10.3390/polym12061381
Sapalidis AA, Karantzis PI, Vairis A, Nitodas SF, Barbe S, Favvas EP. A Study of the Reinforcement Effect of MWCNTs onto Polyimide Flat Sheet Membranes. Polymers. 2020; 12(6):1381. https://doi.org/10.3390/polym12061381
Chicago/Turabian StyleSapalidis, Andreas A., Panagiotis I. Karantzis, Achilles Vairis, Stephanos F. Nitodas, Stéphan Barbe, and Evangelos P. Favvas. 2020. "A Study of the Reinforcement Effect of MWCNTs onto Polyimide Flat Sheet Membranes" Polymers 12, no. 6: 1381. https://doi.org/10.3390/polym12061381
APA StyleSapalidis, A. A., Karantzis, P. I., Vairis, A., Nitodas, S. F., Barbe, S., & Favvas, E. P. (2020). A Study of the Reinforcement Effect of MWCNTs onto Polyimide Flat Sheet Membranes. Polymers, 12(6), 1381. https://doi.org/10.3390/polym12061381