Effect of the Esterification of Starch with a Mixture of Carboxylic Acids from Yarrowia lipolitica Fermentation Broth on Its Selected Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Starch Preparations
2.3. Determination of the Degree of Substitution
2.4. Characteristics of Phase Transitions
2.5. Susceptibility of Starch Esters to the Activity of Amylolytic Enzymes
2.6. Statistical Analysis
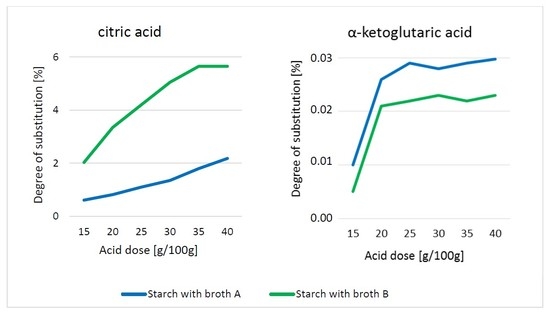
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hong, J.; Zeng, X.A.; Brennan, C.S.; Brennan, M.; Han, Z. Recent Advances in Techniques for Starch Esters and the Applications: A Review. Foods 2016, 5, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zięba, T.; Kapelko, K.; Szumny, A. Effect of preparation method on the properties of potato starch acetates with an equal degree of substitution. Carbohydr. Polym. 2013, 94, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Zięba, T.; Szumny, A.; Kapelko, M. Properties of retrograded and acetylated starch preparations: Part 1. Structure, susceptibility to amylase, and pasting characteristics. LWT Food Sci. Technol. 2011, 44, 1314–1320. [Google Scholar] [CrossRef]
- Zhao, K.; Saleh, A.S.M.; Li, B.; Wu, H.; Liu, Y.; Zhang, G.; Li, W. Effects of conventional and microwave pretreatment acetylation on structural and physicochemical properties of wheat starch. Int. J. Food Sci. Technol. 2018, 53, 2515–2524. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Zięba, T.; Pietrzak, W.; Gryszkin, A. Effect of citric acid esterification conditions on the properties of the obtained resistant starch. Int. J. Food Sci. Technol. 2016, 51, 1647–1654. [Google Scholar] [CrossRef]
- Zdybel, E.; Tomaszewska-Ciosk, E. Modification of starch with succinic acid residues. Przemysł Chemiczny 2015, 94, 1138–1141. (In Polish) [Google Scholar] [CrossRef]
- Chang, P.R.; Qian, D.; Anderson, D.P.; Ma, X. Preparation and properties of the succinic ester of porous starch. Carbohydr. Polym. 2012, 88, 604–608. [Google Scholar] [CrossRef]
- Rudnik, E.; Żukowska, E. Studies on preparation of starch succinate by reactive extrusion. Polimery 2004, 49, 132–134. [Google Scholar] [CrossRef] [Green Version]
- Bhosale, R.; Singhal, R. Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohydr. Polym. 2006, 66, 521–527. [Google Scholar] [CrossRef]
- Passauer, L.; Liebner, F.; Fischer, K. Starch Phosphate Hydrogels. Part I: Synthesis by Mono-phosphorylation and Cross-linking of Starch. Starch 2009, 61, 621–627. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Janas, P. Microwave-assisted preparation of potato starch silicate with silicic acid. Carbohydr. Polym. 2010, 81, 599–606. [Google Scholar] [CrossRef]
- Muliana, H.; Knoop, S.; Picchioni, F.; Jansen, L.P.B.M.; Heeres, H.J. Synthesis of fatty acid starch esters in supercritical carbon dioxide. Carbohydr. Polym. 2010, 82, 346–354. [Google Scholar] [CrossRef]
- Junistia, L.; Sugih, A.K.; Manurung, R.; Picchioni, F.; Jansen, L.P.B.M.; Heeres, H.J. Experimental and Modeling Studies on the Synthesis and Properties of Higher Fatty Esters of Corn Starch. Starch 2009, 61, 69–80. [Google Scholar] [CrossRef]
- Šubarić, D.; Ačkar, D.; Babić, J.; Sakač, N.; Jozinović, A. Modification of wheat starch with succinic acid/acetic anhydride and azelaic acid/acetic anhydride mixtures I. Thermophysical and pasting properties. J. Food Sci. Technol. 2012, 51, 2616–2623. [Google Scholar] [CrossRef] [Green Version]
- Ačkar, D.; Šubarić, D.; Babić, J.; Miličević, B.; Jozinović, A. Modification of wheat starch with succinic acid/acetanhydride and azelaic acid/acetanhydride mixtures. II. Chemical and physical properties. J. Food Sci. Technol. 2012, 51, 1463–1472. [Google Scholar] [CrossRef] [Green Version]
- Ačkar, D.; Šubarić, D.; Babić, J.; Šoštarec, A.; Miličević, B. Modification of barley starch with mixtures of organic dicarboxylic acid and acetanhydride. Technol. Acta 2011, 4, 27–33. [Google Scholar]
- Tomaszewska, L.; Rywińska, A.; Gładkowski, W. Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glicerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, L.; Rakicka, M.; Rymowicz, W.; Rywińska, A. A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res. 2014, 14, 966–976. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska-Hetman, L.; Gołoś, M.; Rymowicz, W.; Rakicka, M.; Rywińska, A. Effect of modification of culture medium containing mixed substrates on a-ketoglutaric acid biosynthesis by Yarrowia lipolitica yeast. Acta Sci. Pol. Biotechnol. 2018, 17, 43–50. (In Polish) [Google Scholar] [CrossRef]
- Cybulski, K. Biosynthesis of pyruvic acid from glycerol by Yarrowia lipolytica yeast. Acta Sci. Pol. Biotechnol. 2013, 12, 5–14. (In Polish) [Google Scholar]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen mass transfer impact on citric acid production by Yarrowia lipolytica from crude glycerol. Biochem. Eng. J. 2016, 110, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Cybulski, K.; Tomaszewska, L.; Rywińska, A. Evaluation of seed culture medium to ketoacids production by Yarrowia lipolytica yeast. Acta Sci. Pol. Biotechnol. 2012, 11, 5–14. (In Polish) [Google Scholar]
- Zdybel, E.; Zięba, T.; Rymowicz, W.; Tomaszewska-Ciosk, E. Organic Acids of the Microbiological Post-Culture Medium as Substrates to be Used for Starch Modification. Polymers 2019, 11, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zięba, T.; Kapelko-Żeberska, M.; Gryszkin, A.; Wilczak, A.; Raszewski, B.; Spychaj, R. Effect of the Botanical Origin on Properties of RS3/4 Type Resistant Starch. Polymers 2019, 11, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapelko, M.; Zięba, T.; Michalski, A.; Gryszkin, A. Effect of cross-linking degree on selected properties of retrograded starch adipate. Food Chem. 2015, 167, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Rymowicz, W. Continuous production of citric acid from raw glycerol by Yarrowia lipolytica in cell recycle cultivation. Chem. Pap. 2011, 65, 119–123. [Google Scholar] [CrossRef]
- Rakicka, M.; Rywińska, A.; Rymowicz, W. Purification of erythritol by crystallization. Acta Sci. Pol. 2017, 16, 5–18. (In Polish) [Google Scholar]
- Mei, J.Q.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effects of citric acid esterification on digestibility, structural and physicochemical properties of cassava starch. Food Chem. 2015, 187, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, A.N.; Moorthy, S.N.; Sreekumar, J.N.; Rajasekharan, K.N. Studies on the properties of citrate derivatives of cassava (Manihot esculenta Crantz) starch synthesized by microwave technique. J. Sci. Food Agric. 2007, 87, 871–879. [Google Scholar] [CrossRef]
- Olsson, E.; Hedenqvist, M.S.; Johansson, C.; Järnström, L. Influence of citric acid and curing on moisture sorption, diffusion and permeability of starch films. Carboh. Polym. 2013, 94, 765–772. [Google Scholar] [CrossRef]
- Ye, J.; Luo, S.; Huang, A.; Chen, J.; Liu, C.; McClements, J. Synthesis and characterization of citric acid esterified rice starch by reactive extrusion: A new method of producing resistant starch. Food Hydrocoll. 2019, 92, 135–142. [Google Scholar] [CrossRef]
- Kim, H.S.; Min, S.C. Effects of microwave-discharged cold plasma on synthesis and characteristics of citrate derivatives of corn starch granules. Food Sci. Biotechnol. 2017, 26, 697–706. [Google Scholar] [CrossRef]
- Leszczyński, W. Resistant starch—Classification, structure, production. Pol. J. Food Nutr. Sci. 2004, 13, 37–50. [Google Scholar]
- Yoo, H.J.; Kim, H.R.; Choi, S.J.; Park, C.-S.; Moon, T.W. Characterisation of low-digestible starch fractions isolated from amylosucrase-modified waxy corn starch. Int. J. Food Sci. Technol. 2018, 53, 557–563. [Google Scholar] [CrossRef]
- Shin, S.I.; Lee, C.J.; Kim, M.J.; Choi, S.J.; Choi, H.J.; Kim, Y.; Moon, T.W. Structural characteristics of low-glycemic response rice starch produced by citric acid treatment. Carboh. Polym. 2009, 78, 588–595. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Q. Development and physicochemical characterization of new resistant citrate starch from different corn starches. Starch 2004, 56, 364–370. [Google Scholar] [CrossRef]
- Wepner, B.; Berghofer, E.; Miesenberger, E.; Tiefenbacher, K.; Ng, P.N.K.; Lansing, E. Citrate starch–application as resistant starch in different food systems. Starch 1999, 51, 354–361. [Google Scholar] [CrossRef]
- Sekine, M.; Otobe, K.; Sugiyama, J.; Kawamura, Y. Effects of heating, vacuum drying and steeping on gelatinization properties and dynamic viscoelasticity of various starches. Starch 2000, 52, 398–405. [Google Scholar] [CrossRef]
- Butt, N.A.; Ali, T.M.; Hasnain, A. Rheological characterization of cold water soluble rice (Oryza sativa) starch lactates and citrates prepared via alcoholic-alkaline method. Int. J. Biol. Macromol. 2019, 123, 558–568. [Google Scholar] [CrossRef]
- Srikaeo, K.; Hao, P.T.; Lerdluksamee, C. Effects of heating temperatures and acid concentrations on physicochemical properties and starch digestibility of citric acid esterified tapioca starches. Starch 2019, 71. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.-Q.; Wu, M.; Zhang, H.; Wang, J.-H. Retrogradation of partially gelatinised potato starch prepared by ball milling. Int. J. Food Sci. Technol. 2018, 53, 1065–1071. [Google Scholar] [CrossRef]




| Starch Preparation | Acid Dose (g/100 g) | Specific Heat of Phase Transition (J/g) | Temperature of Onset of Phase Transition (°C) | Temperature of Maximum of Phase Transition (°C) | Temperature of End of Phase Transition (°C) |
|---|---|---|---|---|---|
| Starch without broth | 0 | 11.62 ± 0.25 k | 54.83 ± 0.64 g | 61.27 ± 0.49 i | 69.87 ± 0.58 h |
| Starch with broth A | 15 | 9.97 ± 0.20 j | 48.66 ± 0.52 f | 56.00 ± 0.42 h | 68.02 ± 0.58 g |
| 20 | 8.64 ± 0.21 i | 43.91 ± 0.52 e | 53.67 ± 0.48 g | 65.90 ± 0.60 f | |
| 25 | 7.80 ± 0.21 h | 41.84 ± 0.48 bc | 51.02 ± 0.30 f | 62.63 ± 0.46 e | |
| 30 | 7.02 ± 0.19 g | 41.64 ± 0.41 b | 50.84 ± 0.33 ef | 62.80 ± 0.42 e | |
| 35 | 6.92 ± 0.29 g | 41.28 ± 0.53 ab | 50.42 ± 0.48 de | 62.86 ± 0.58 e | |
| 40 | 5.67 ± 0.40 f | 40.88 ± 0.55 a | 49.94 ± 0.50 cd | 61.52 ± 0.55 d | |
| Starch with broth B | 15 | 5.18 ± 0.22 e | 43.95 ± 0.40 e | 53.79 ± 0.33 g | 63.17 ± 0.39 e |
| 20 | 3.89 ± 0.31 d | 42.68 ± 0.61 d | 49.82 ± 0.58 c | 60.98 ± 0.58 d | |
| 25 | 3.01 ± 0.38 c | 42.64 ± 0.68 d | 48.02 ± 0.52 b | 58.14 ± 0.58 c | |
| 30 | 1.62 ± 0.19 b | 42.44 ± 0.45 cd | 48.28 ± 0.33 b | 57.23 ± 0.40 b | |
| 35 | 1.29 ± 0.19 a | 41.74 ± 0.47 b | 47.14 ± 0.33 a | 55.20 ± 0.45 a | |
| 40 | 1.29 ± 0.21 a | 41.37 ± 0.47 ab | 47.21 ± 0.40 a | 55.36 ± 0.45 a | |
| LSD | 0.32 | 0.64 | 0.50 | 0.62 | |
| Starch Preparation | Modification Temperature (°C) | Specific Heat of Phase Transition (J/g) | Temperature of Onset of Phase Transition (°C) | Temperature of Maximum of Phase Transition (°C) | Temperature of End of Phase Transition (°C) |
|---|---|---|---|---|---|
| Starch without broth | 120 | 11.72 ± 0.28 h | 54.79 ± 0.60 g | 61.30 ± 0.52 g | 70.10 ± 0.58 j |
| 130 | 11.62 ± 0.25 h | 54.83 ± 0.64 g | 61.27 ± 0.49 g | 69.87 ± 0.58 ij | |
| 140 | 10.97 ± 0.36 g | 54.46 ± 0.58 g | 61.13 ± 0.44 fg | 69.38 ± 0.45 hi | |
| 150 | 9.88 ± 0.34 f | 53.60 ± 0.55 f | 60.74 ± 0.40 f | 69.25 ± 0.48 h | |
| Starch with broth A | 120 | 9.12 ± 0.22 e | 45.18 ± 0.50 e | 55.12 ± 0.46 e | 67.54 ± 0.56 g |
| 130 | 8.64 ± 0.21 d | 43.91 ± 0.52 d | 53.67 ± 0.48 d | 65.90 ± 0.60 f | |
| 140 | 5.94 ± 0.40 c | 40.54 ± 0.56 b | 50.02 ± 0.48 b | 62.64 ± 0.55 d | |
| 150 | 5.89 ± 0.41 c | 40.12 ± 0.54 ab | 49.80 ± 0.42 b | 58.81 ± 0.54 b | |
| Starch with broth B | 120 | 4.01 ± 0.30 b | 44.92 ± 0.60 e | 51.62 ± 0.59 c | 63.74 ± 0.58 e |
| 130 | 3.89 ± 0.31 b | 42.68 ± 0.61 c | 49.82 ± 0.58 b | 60.98 ± 0.58 c | |
| 140 | 3.12 ± 0.38 a | 39.74 ± 0.56 a | 46.20 ± 0.56 a | 55.50 ± 0.60 a | |
| 150 | 2.74 ± 0.31 a | 39.85 ± 0.60 a | 45.81 ± 0.58 a | 54.93 ± 0.60 a | |
| LSD | 0.38 | 0.58 | 0.50 | 0.58 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdybel, E.; Zięba, T.; Tomaszewska-Ciosk, E.; Rymowicz, W. Effect of the Esterification of Starch with a Mixture of Carboxylic Acids from Yarrowia lipolitica Fermentation Broth on Its Selected Properties. Polymers 2020, 12, 1383. https://doi.org/10.3390/polym12061383
Zdybel E, Zięba T, Tomaszewska-Ciosk E, Rymowicz W. Effect of the Esterification of Starch with a Mixture of Carboxylic Acids from Yarrowia lipolitica Fermentation Broth on Its Selected Properties. Polymers. 2020; 12(6):1383. https://doi.org/10.3390/polym12061383
Chicago/Turabian StyleZdybel, Ewa, Tomasz Zięba, Ewa Tomaszewska-Ciosk, and Waldemar Rymowicz. 2020. "Effect of the Esterification of Starch with a Mixture of Carboxylic Acids from Yarrowia lipolitica Fermentation Broth on Its Selected Properties" Polymers 12, no. 6: 1383. https://doi.org/10.3390/polym12061383
APA StyleZdybel, E., Zięba, T., Tomaszewska-Ciosk, E., & Rymowicz, W. (2020). Effect of the Esterification of Starch with a Mixture of Carboxylic Acids from Yarrowia lipolitica Fermentation Broth on Its Selected Properties. Polymers, 12(6), 1383. https://doi.org/10.3390/polym12061383